Translating laboratory discoveries to clinical care for patients with cancer is a difficult process. Much of the effort is carried by researchers who are also trained as physicians, especially a […]
Clinical research organizations face a seemingly bewildering array of financial and regulatory demands, but there’s light at the end of the tunnel for those who proactively develop compliance programs, says […]
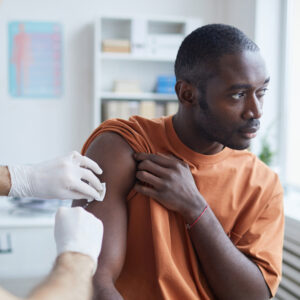
A new study confirms what many suspected about a tumultuous year for conducting clinical trials: Patient recruitment and retention dropped across the industry in early 2020, due to global quarantines […]
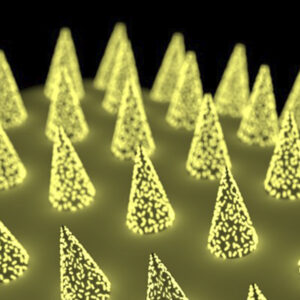
Blood draws are no fun. They hurt. Veins can burst, or even roll—like they’re trying to avoid the needle, too. However, clinical trial protocols often include instructions for regular blood […]
Studies examining the effectiveness of treatments for COVID-19 often do not include the very populations hardest hit by the disease, according to a new review by University of Chicago Medicine […]