Clinical Researcher—November 2021 (Volume 35, Issue 8)
PEER REVIEWED
Carol Brooks, BHS, ACRP-CP; Robin Ryan, MPH, CCRP
The International Council for Harmonization (ICH) Guideline for Good Clinical Practice (GCP) states, “Each individual involved in conducting a [clinical] trial should be qualified by education, training, and experience to perform his or her respective task(s).”{1} It is of great importance that the trial can be reconstructed as it happened. An external observer should be able to confirm that the current protocol was followed, the data and information collected were accurate, and the staff conducting the trial were properly qualified and trained to do so. Proper documentation will provide an audit trail that will validate the trial if, and when, required.
Protocol-specific training can delay the activation of a study as well as a potential subject’s enrollment. Systemizing and documenting staff training for multidisciplinary trials is challenging. For those cooperative group studies with multiple investigators and other study staff, it can especially be quite challenging. Schedules are full and often are geographically scattered. Accommodating time to schedule an in-person group training or finding time for individuals to complete self-training is difficult. For some individuals, completion of training can be overlooked since it is not necessarily a top priority for busy clinicians.
Children’s Mercy Hospital is a pediatric medical center located in Kansas City, Mo. that integrates holistic care, translational research, breakthrough innovation, and medical education to provide care for those 21 years old and below. The not-for-profit hospital has received national recognition from U.S. News & World Report in 10 pediatric specialties.{2} Its mission is to “transform the health, well-being, and potential of children, with unwavering compassion for those most vulnerable.” The research program at the hospital includes nearly 100 physicians and scientists actively participating in research studies. Research is especially important in the oncology section, as most of the active trials are treatment options for patients to whom they could be beneficial.
Past Training Methods at Children’s Mercy
Prior to 2018, the Oncology Section at Children’s Mercy did not have a systematic method of training and documenting protocol-specific training compliance. Pediatric oncology research includes numerous treatment and non-treatment protocols for the various types of cancer. Children’s Oncology Group (COG) is the main consortium that sponsors research in the Oncology Section. Efforts to maintain current training and documentation among our COG team were difficult, as we have more than 30 active COG protocols and more than 60 COG site personnel to train. While in-person trainings and e-mail documentation were utilized, these were not consistent or easily validated. Additionally, e-mail created extra work for the coordinator—to keep track of the progress of each team member and forwarding reminders for those pending completion.
In 2017, the research team within the Division of Hematology/Oncology/BMT was restructured to create a separate regulatory coordinator role. Part of the rationale for this separation of regulatory work from patient-facing study coordination was to be more rigorous with training and documentation thereof. The oncology research team wanted to identify an efficient way to distribute and track completion of protocol-specific training for study team members.
An informal review of training options was undertaken by the institution’s research leadership. This involved looking at available technology for possibly accommodating the needs. In 2018, it was decided to determine if REDCap could effectively document training.
Elements of REDCap
REDCap (Research Electronic Data Capture) is a secure web application from Vanderbilt University that can be used to build and manage online surveys. There is no special software installation needed for utilization. If institutions have this application available, there are no fees charged to use it. This web application is versatile and is used widely among different fields. At Children’s Mercy, REDCap was already being used for databases, survey tools, research data collection, and e-consenting. If the team could utilize REDCap for training, it would provide an easily available option.
Rationales for choosing REDCap as a training platform included the fact that the survey tool is able to track the progress of completion. Using the survey feature, the regulatory coordinator can send protocol training to the identified study team. Survey recipients do not need special access to REDCap to get a link to review the training and attest to completion. For each personnel added to the participant list, REDCap will show if they have responded to the survey. REDCap will also show if there will be an upcoming invitation that is scheduled to be sent.
Further, REDCap can be set up to send automatic e-mail reminders. Keeping up with manually reminding delinquent personnel to complete training is time consuming. With REDCap, the frequency of the reminders, date, and time are all customizable.
REDCap also features an application that can generate a report of those who have submitted the surveys which can then be used as a training log. The reports are customizable, but can contain the date training was complete, timestamp, names, e-mail, and/or signatures (see Figure 1).
Figure 1: Example of a REDCap-Generated Training Log

Development of a Training Template
There are a few important items that should be included in the survey for training. At our site, initial training includes a PowerPoint module reviewing the important aspects of the protocol. This PowerPoint is usually provided by the sponsor, but, if one is not available, it can be created by the site’s principal investigator (PI). Attaching the written protocol and/or manuals for reference and as supplemental material is always a good idea.
For amendment training, the survey includes a summary of changes, an updated PowerPoint module, and the newly amended protocol. Within the survey, a section is included to attest that review of training materials has been completed, a block to include questions and/or concerns, a name stamp, an e-mail address stamp, and a date stamp (see Figure 2 for the training template).
Implementation of REDCap for the Oncology Team
Implementing this new format for the COG team was challenging due to the large number of team members. A simple workflow, however, was established and made to fit to accommodate the various COG protocols.
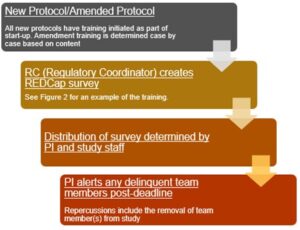
As new protocols are activated, the regulatory coordinator pushes out initial protocol training surveys to appropriate team members. The team member roster is determined at the time of protocol start-up. Required amendment training is determined based on content of the amendment.
Any amendment changing therapy, eligibility, or other major changes to the protocol will be forwarded as a REDCap survey training by the regulatory coordinator. All training surveys include a deadline date for completion.
As the training deadlines pass, the regulatory coordinator will communicate to the PI the list of delinquent team members. The PI alerts these individuals, and if failure to comply continues, repercussions will include the removal of the team member(s) from the study. Figure 3 is a representation of the workflow explained above.
| Figure 2: REDCap Training Template
As a person involved in [DEPARTMENT] research, you are asked to review the training for [STUDY TITLE] Thank you! |
| Because you participate or may participate in the conduct of [DEPARTMENT] trials at Children’s Mercy, you are required to complete this training. Please review the [LIST MATERIALS SUCH AS PROTOCOL OR SLIDES] attached. At the end of this survey, you will be required to attest to having completed the review.
If you have any questions or comments, there is also a place to note that. |
| [PROTOCOL Number/Title] Required Training
Attach training slides |
| Attach protocol here |
| ATTESTATION
I have reviewed the [PROTOCOL #] training Yes No [MATERIALS]. * must provide value Reset |
| After reviewing the [MATERIALS] I have no questions or comments
* must provide value I have questions or comments Reset |
| SIGN AND SUBMIT
Name * must provide value First and Last |
| Please add your e-mail address to attest to your completion. You will not be able to submit this form without this completed.
* must provide value |
| Date Today M-D-Y
* must provide value
|
| Provide Signature:
Add signature * must provide value |
|
Submit |
Figure 3: Representation of Workflow for Protocol Training
When this training system was implemented, it was with the knowledge that there would be a learning curve and that turnaround for completion was going to be less than satisfactory. Numerous auto-generated reminders were necessary to get people “onboard.” As team members became more familiar with the process, compliance improved dramatically.
Organization for the regulatory coordinator with multiple studies and amendments also required a system. With multiple training surveys in process at once, and most deadlines for completion in a four- week range, a tracker of active surveys was developed. Another useful tool is a master study team list. As the studies the lead author is involved in can include as many as 65 people from multiple disciplinary teams at one time, a spreadsheet listing the personnel, their e-mails, and roles comes in handy. With this, all that is needed is to copy and paste e-mail addresses into the study participant list when surveys are created. The lead author also created a document listing e-mail templates for when she sends REDCap survey invitations or reminders, and has developed e-mail templates for initial training for new studies and applicable amendment training.
Dissemination Within the Institution and to Other Institutions
Once our process was established, it was shared at the Fall 2018 COG Poster Session. The lead author has met numerous people interested in learning more about using REDCap surveys for training. Upon follow-up a year later to each inquiry, three (from the University of Florida, CancerCare Manitoba, and Dana- Farber Cancer Institute) have expressed gratitude and intend on using REDCap as the main platform for training documentation.
Within Children’s Mercy, the REDCap training process is now being used within other sections of the hospital with resources on how to create the successful workflow shared with research teams that inquire. The institutional Research Quality team recommends the REDCap training system to other teams during monitoring visits and provides contact information to learn more.
Conclusion
Attributes for good documentation as described by the U.S. Food and Drug Administration are attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available.{3} REDCap meets all these attributes and creates an audit trail of documentation reflecting compliance with GCP. REDCap has become the main tool used for providing and documenting training at the Children’s Mercy Hospital—Oncology Section, as this effort has been very effective and user-friendly.
References
- See ICH GCP E6(R2) at https://www.ich.org/page/efficacy-guidelines.
- https://health.usnews.com/best-hospitals/area/mo/childrens-mercy-hospitals-and-clinics-6630340/pediatric-rankings/cancer
- https://conductscience.com/portfolio/alcoa-c/

Carol Brooks, BHS, ACRP-CP, (cmbrooks@cmh.edu) is a Clinical Trials Coordinator (Regulatory) for oncology/bone marrow transplant studies with Children’s Mercy Hospital in Kansas City, Mo.

Robin Ryan, MPH, CCRP, is Research Program Director, Division of Hematology/Oncology/Stem Cell Transplant, Children’s Mercy Hospital.



