A study involving virtual rather than real patients was as effective as traditional clinical trials in evaluating a medical device used to treat brain aneurysms, according to new research.
The findings are proof of concept for what are called in-silico trials, where instead of recruiting people to a real-life clinical trial, researchers build digital simulations of patient groups, loosely akin to the way virtual populations are built in The Sims computer game. Such trials could revolutionize the way clinical trials are conducted, reducing the time and costs of getting new medical devices and medicines developed, while also reducing human and animal harm in testing.
The virtual patient populations are developed from clinical databases to reflect age, sex, and ethnicity. They also simulate the way disease affects the human body; for example, the interactions between anatomy, physics, physiology, and blood biochemistry. Those simulations are then used to model the impact of therapies and interventions.
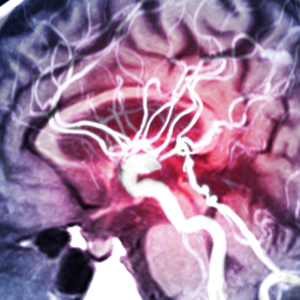
The international research, led by the University of Leeds and reported in the journal Nature Communications, investigated whether an in-silico trial could replicate the results of three real-life clinical trials that assessed the effectiveness of a device called a flow diverter, used in the treatment of brain aneurysms, in which the wall of a blood vessel weakens and begins to bulge.
The researchers built a virtual population of 82 cases using real patient data drawn from clinical databases, ensuring that the anonymized virtual patients closely resembled the patients used in real flow diverter clinical trials in terms of age, sex, and aneurysm characteristics. They then built a computational model that analyzed how the implanted device would affect blood flow in each of the virtual patients. They were able to study different physiological conditions for each patient, such as normal and high blood pressure, and perform analyses on patient sub-groups, such as those with large aneurysms or aneurysms with a branch vessel.
The results of the virtual trial predicted that 82.9% of the virtual patients with normal blood pressure would be successfully treated with a flow diverter. In the three traditional clinical trials, the percentage of people successfully treated was 86.8%, 74.8%, and 76.8%, thus showing that the virtual trial replicated the traditional clinical trials results.
According to the project’s supervisor, the current approach to improve scientific understanding of new medical devices is slow, as conventional trials can easily take five to eight years from their design to completion. In-silico trials, he says, could reduce this period to less than six months in some circumstances, making knowledge and therapeutic technologies safer and more promptly available to clinicians and patients.
The research involved an international collaboration of scientists from Leeds, the University of Oxford, Radbound University Medical Centre in the Netherlands, and KU Leuven in Belgium.
Edited by Gary Cramer