Among the latest clinical research enterprise initiatives making waves at the recent DIA 2023 meeting in Boston was word of a new and comprehensive toolkit for use by institutional review boards (IRBs)/ethics committees (ECs) to standardize decentralized clinical trial (DCT) ethics review. Its creators say that the first-of-its-kind toolkit provides a common framework, tools, and best practices for uniform ethical review and approval, as well as a roadmap for the ethical conduct of DCTs. Ultimately, the members of the task force behind the toolkit say that such standardization will simplify, streamline, and speed the IRB/EC process—a key step toward more efficient, patient-centered research execution.
Responding to questions posed by ACRP, Dr. Barbara Bierer, Professor of Medicine at Harvard Medical School and Faculty Director of the Multi-Regional Clinical Trials (MRCT) Center of Brigham & Women’s Hospital and Harvard, and Dr. Pamela Tenaerts, Chief Scientific Officer at Medable, below provide some background on how the toolkit came about and how it could evolve over time. The project was undertaken by a task force consisting of patients, patient advocates, and representatives of the MRCT Center, Medable, the U.S. Food and Drug Administration (FDA), the U.S. Office for Human Research Protections, and various IRBs/ECs and study sites—all collaborating for more than 18 months to create the new DCT IRB/EC Toolkit.
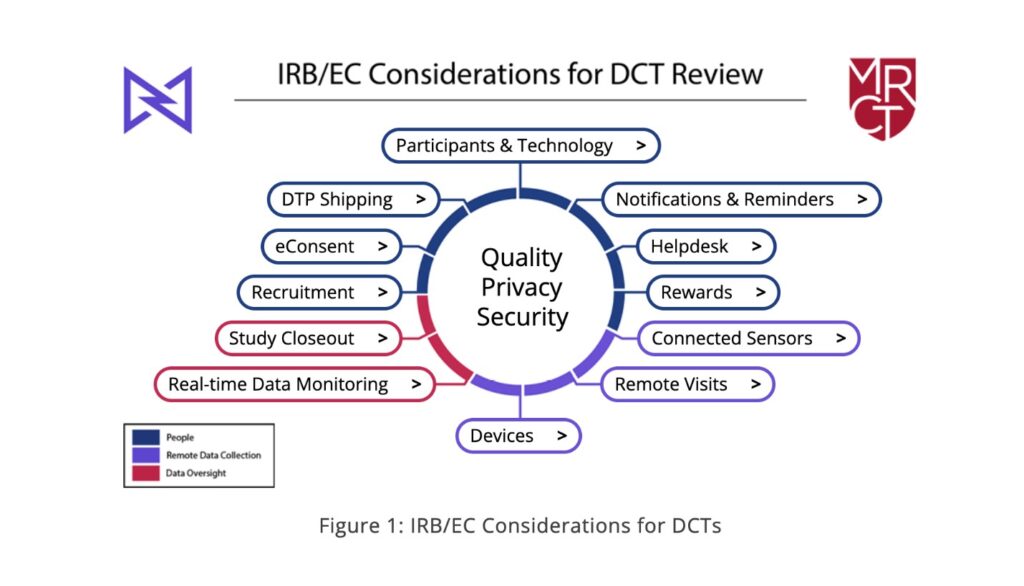
Members of the task force followed the participant journey through a typical trial and approached each element of it separately as they developed the toolkit. The end product encompasses 12 guides organized around three themes of people, data collection, and data oversight (see Figure 1)—addressing each common element of a decentralized trial. These range from electronic consent (eConsent), electronic clinical/patient-reported outcomes assessment (eCOA/ePRO), wearable devices, remote telehealth visits, and more.
Importantly, Drs. Bierer and Tenaerts say that the task force created recommendations and tools that apply to both U.S. and global trials and to all study site types and designs. They advocate for case-by-case protocol review using a principled perspective, appreciating that some considerations may not be applicable to all study sites. For example, they note, a protocol that identifies participants through an in-patient interaction will not be fully decentralized, and, conversely, a protocol that intends to validate a mobile technology may never require the participant to be seen in a research facility.
Q: What were the “toughest nuts to crack” for the task force in terms of deciding how to standardize the many different facets of study review/approval in the case of DCTs?

Bierer: Aspects of decentralization in clinical trials vary from trial to trial—from a simple lab or imaging study locally or a home health visit to a fully decentralized experience where the participant never appears at a traditional research site. It was, therefore, difficult to balance the appropriate degree of detailed oversight to recommend. We opted to promote a risk-based approach based on each specific decentralized element of a trial, with a constant view of protecting the health, welfare, and safety of participants and attention to their privacy and confidentiality.
 Tenaerts: The privacy and security questions and the need for their deliberation came up in almost every element of decentralized trials we discussed. To facilitate those discussions, we created considerations specific to every decentralized element and created a summary document that separates them out. The discussions within each of the 12 sections of the toolkit revealed layers of questions, indicating the power of a multi-stakeholder task force to identify salient considerations for ethical reviews.
Tenaerts: The privacy and security questions and the need for their deliberation came up in almost every element of decentralized trials we discussed. To facilitate those discussions, we created considerations specific to every decentralized element and created a summary document that separates them out. The discussions within each of the 12 sections of the toolkit revealed layers of questions, indicating the power of a multi-stakeholder task force to identify salient considerations for ethical reviews.
Q: Is this toolkit equally applicable to the work that goes into decisions from local (internal) and central (external/for-profit) IRB/EC services?
Bierer: We consider these recommendations, considerations, and tools to be applicable to all ethics committees, locally or globally, whether reviewed by a single ethics committee or multiple ethics committees. They are also intended to facilitate appropriate submissions by the protocol writer(s) to the ethics committees so that all salient documentation related to the decentralized methods can be included. We hope that outlining common requirements will facilitate appropriate ethical deliberation, focus on key issues, and decrease the time delays incurred when ethics committees request additional information and documentation.
Q: Can you share any key insights that are reflected in the end product that came from patients? From sites?
Bierer: We convened a multi-stakeholder task force that included representatives from patients, patient advocates, investigators, and sites as well as other stakeholders such as IRBs, regulators, and industry. The themes and recommendations reflect the collective opinions of the varied members of the task force. In addition, there were some topics, such as eConsent, eCOA, and Helpdesk, where we brought in content experts from Medable who had insights into participant and site experience and feedback and could provide the expertise necessary.
Q: Can you foresee a 2.0 version of the toolkit eventually being available after sufficient experience and feedback from the launch version’s users?
Tenaerts: The use of decentralized approaches is increasing and changing. For example, the European Medicines Agency reflection paper on DCTs and the FDA Draft DCT Guidance were both released after the task force discussions were complete. We hope that our recommendations are utilized and that we receive feedback and suggestions, which can be used to make periodic updates in the future. This is a rapidly evolving area, and we certainly anticipate not only a 2.0 version but additional versions—and other tools and resources—to be developed over time.
Editor: Gary Cramer




