Clinical Researcher—February 2020 (Volume 34, Issue 2)
FORM & FUNCTION
Lindsay McNair, MD, MPH, MSB
For many years, the randomized, parallel-group, double-blinded clinical trial was considered the optimal study design to assess the efficacy and safety of new investigational therapies. As the science behind therapeutic interventions has deepened and grown, the clinical trial designs through which those interventions can be best tested have evolved as well.
One of the therapeutic areas in which this has been seen most dramatically is oncology. “Precision medicine” includes the development of agents targeted to specific molecular profiles, including specific genetic mutations that may be driving cancer growth. These genetic mutations may appear in more than one type of cancer. For example, cancers with the TRK fusion protein may be found in the colon, breast, or lung. To study therapies directed against these specific abnormalities, it may make sense to include anyone with the target abnormality in the trial population, regardless of the location of their cancer.
Basket Clinical Trials
This is the premise of a “basket” trial design: that patients from different disease groups or subgroups, such as those with different types of cancer, are identified within those groups based on the presence of a specific factor. Patients with that factor who agree to be study participants are grouped together in one cohort, or “basket,” to receive an experimental product which is designed to work, or to work best, in that specific category of disease (see Figure 1).
Figure 1: A Visualization of the Basket Trial Design
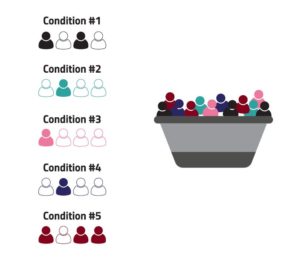
Basket designs are intended to study a single investigational regimen in a number of different diseases or disease subtypes. The application of this idea is not just conceptual; the first drug to receive approval from the U.S. Food and Drug Administration (FDA) for a “tissue-agnostic” indication was pembrolizumab, for the treatment of specific patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors, in 2018.{1} Later that year, FDA approved larotrectinib for the treatment of solid tumors with a NTRK fusion mutation.{2}
Basket trials are one of a set of novel clinical trial designs that are generally referred to as “master” protocol designs, which are intended to answer multiple questions in one study. Master protocol designs include platform trials (studies designed to assess multiple therapeutic agents in the same study population, amending the protocol design when the ongoing data interpretation indicates that either a futility or efficacy threshold is reached), and umbrella trials (looking at multiple investigational agents against the same disease as subgroups of one overall study population).{3}
As with all trial designs, each of the “master” designs has settings in which it may be optimally useful. Basket trial designs are often used as early studies in the development of a new agent. Although theoretically all tumors with the same mutation would respond in the same way to an agent targeted against the effect of that mutation, in practice that doesn’t always occur. Looking at patients with different types of cancer can help identify the most responsive tumor types, providing direction for future development.
In this way, the basket concept is not very different from the traditional early studies in which a new agent may be tested in a population that includes participants with “any solid tumor” and the goal of identifying early efficacy signals while collecting safety data. However, in the basket design the study population is enriched by including only those participants with markers that make them most likely to respond to the intervention.
While the basic concept of a basket trial is fairly simple, as with all conceptual study designs, the application in practice can become much more complex. In some studies, the agent being tested may have potential efficacy against more than one target mutation, so that there is more than one mutation-specific cohort, such as in the European Organization for the Research and Treatment of Cancer (EORTC) CREATE trial of crizotinib.{4}
Some authors report that the term “basket” has come to be applied fairly broadly to any study design in which a targeted therapy (or therapies) is being tested in a distinct disease subtype or types, but where the actual designs can vary from a single cohort of participants to what is effectively a series of distinct Phase II studies.{5}
While basket trial designs are almost exclusively discussed as oncology trials, because of the large number of oncology products that target specific molecular markers that lend themselves to this design, the general concept could certainly be applicable to other therapeutic areas as well. For example, an immuno-modulating agent could be tested in an early trial in participants who have a variety of auto-immune conditions rather than in one narrow indication.
Operational Considerations
In planning trials that employ a basket design, it’s important to look at the operational implications, where the advent of master protocol designs has brought changes as well. These include the use of screening platforms to allow potential participants to be screened for multiple studies at the same time.
Further, the centralization of study governance committees and institutional review board oversight enables more efficient study conduct and streamlined communication and decision-making pathways. These are especially important factors when the studies may include adaptive elements that require real-time assessment of incoming information and rapid responses to those data.
However, despite the operational advances in some areas, master protocols may still be challenging to conduct. In most institutions, clinical divisions and administrative infrastructure are designed based on disease type or location: the breast oncology department, the gastrointestinal oncology department, etc. Research administration is often parallel to this, so that research team staffing, facilities, budgets, and other considerations are allocated and managed by disease-based divisions.
In disease-location agnostic study, this structure needs to be re-invented. Study coordinators may have to work together or to work across departments.
Summary
As therapeutic agents evolve and our understanding and ability to utilize precision medicine grows, the study designs that we use to test the safety and efficacy of these novel therapies must evolve as well. While there is still a place for the standard randomized, parallel-group clinical trial, designs such as for basket trials allow the enrichment of study populations for specific markers to which therapies are targeted.
Taking advantage of both study design and operational advances in the way we conduct clinical research will help us to answer research questions with efficacy and scientific rigor, allowing us to identify promising therapies more quickly and to move them forward toward the clinic.
References
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication
- Mullard, A. 2019. FDA approves landmark tissue-agnostic cancer drug. Nat Rev Drug Discov18(7). https://doi.org/10.1038/nrd.2018.226
- Woodcock J, LaVange LM. 2017. Master protocols to study multiple therapies, multiple diseases, or both. NEJM 377:62–70. doi: 10.1056/NEJMra1510062
- CREATE: Cross-tumoral Phase 2 with Crizotinib. https://clinicaltrials.gov/ct2/show/NCT01524926
- Cunanan KM, Gonen M, Shen R, et al. 2017. Basket trials in oncology: a trade-off between complexity and efficiency. J Clin Oncol 35(3):271–3. doi:10.1200/JCO.2016.69.9751

Lindsay McNair, MD, MPH, MSB, is Chief Medical Officer at WCG (WIRB-Copernicus Group).



