A new guidance from the U.S. Food and Drug Administration (FDA) provides recommendations and updated information to help sponsors comply with the expedited safety reporting requirements for human drug and […]
The COVID-19 pandemic “exposed severe disparities in our healthcare system,” including the relative lack of diversity in the clinical trial patient population, Bashar Shihabuddin, MD, MS, a professor of emergency medicine […]
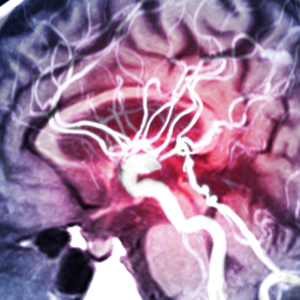
A study involving virtual rather than real patients was as effective as traditional clinical trials in evaluating a medical device used to treat brain aneurysms, according to new research. The […]
Data captured in one segment of the National Cardiovascular Data Registry (NCDR) suite of registries is similar in quality, depth, and granularity when compared to data captured through clinical trials, […]
Clinical trial practitioners should be aware of a new European Union (EU) Clinical Trials Regulation (Regulation No 536/2014) that will require lay (plain language) summaries for every clinical trial conducted […]