Clinical Researcher—February 2022 (Volume 36, Issue 1)
SITES & SPONSORS
Anusha Shetty
Nearly one out of every 10 clinical trials launched never enrolls a single patient.{1} This is costly and time-consuming for all stakeholders, yet a failure to meet patient recruitment targets is one of the most common reasons clinical trials are stopped or delayed. Of the suspended studies between 2011 and 2021, 30% were due to a low number of participants.{2}
Starting trials with the right clinical research sites can drive better patient recruitment, streamline execution, and improve study quality. To help clinical leaders develop strategies for efficient site feasibility and selection, we’ll explore the key challenges the industry faces, areas in need of improvement, the role of technology, and what the future holds for study start-up.
Poor Site Engagement is Holding Trials Back
Selecting the right research site is vital to the success of a study, but finding a partner that can maximize patient enrollment has been an industry-wide issue since 86% of clinical trials don’t meet recruitment targets within specified periods.{3} The first step to establishing a successful site engagement strategy is understanding key study start-up challenges and how they impact trial outcomes. Let’s consider four of these challenges, just to warm ourselves up to the topic.
Lengthy and complex questionnaire process. Feasibility surveys are typically long (including approximately 40 to 75 questions) and many of the sites’ responses are applicable across studies, such as the total number of exam rooms. Yet, site responses aren’t being reused or pre-populated on subsequent questionnaires. This becomes tedious and inefficient for site staff, many of whom are short on resources.
Siloed information. After completing a successful study, many sponsors and contract research organizations (CROs) don’t save and reuse the data captured about investigators and their sites. While valuable data about a site’s performance exist in the hands of individuals on spreadsheets and in e-mails, there is no easy way to query and leverage this information for future studies. Without a reliable database and a central repository of site profiles, everyone loses costly time, including principal investigators and their staff.
Site accessibility and availability. Selecting a site that has delivered in the past provides a sense of security, but this approach can be problematic because it narrows the reach of the proposed research. By not conducting thorough site selection, sponsors and CROs can miss out on talented investigators who don’t have the resources to promote their areas of expertise.
Add on the intense competition for sites and the pressure to engage quickly (sometimes within two weeks), and it might seem like using the site you know is the best option. However, this isn’t always a best practice that delivers results, since it limits access to new patient populations in previously untapped areas.
Too many systems for sites. Sponsors and CROs have different software systems and security, privacy, and regulatory standards for every study, placing an additional burden on already resource-strapped sites. Many opt to use manual or paper-based processes to overcome this challenge, increasing quality and compliance risks because investigators can’t use the technology provided for a trial.
Enabling Seamless Study Start-Up
It’s time to reimagine site engagement and implement new strategies that make it easier for sponsors/CROs and sites to work with one another across multiple studies. To begin this transformation, organizations should prioritize evaluating and adopting modern study start-up technology, especially since 81% of sponsors and CROs still use spreadsheets to manage start-up processes.{4} A shift in strategy and use of purpose-built study start-up applications can help drive long-lasting, positive change. Here are three steps companies can take now to enable a more seamless trial tomorrow.
Establish a data-driven site identification strategy. Leverage public domain data and internal resources to collect critical data about site capabilities. Information should be stored in a format that is easy to access and analyze. Key examples are details like after-hours contact information and specific site successes and failures.
With this information readily available, sponsors and CROs can efficiently conduct queries and make more informed decisions. Figure 1 provides an example of what can be accomplished with better site performance data.
Figure 1: Better site performance data lead to more informed decisions in study start-up.
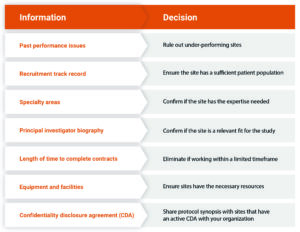
Simplify feasibility questionnaires. Capture precise data about a site with a questionnaire that delivers valuable insight into a site’s suitability for the upcoming study. Instead of how many patients are in your database, edit the question to how many of your patients have this specific disease. Framing the questions to draw out detailed information will help companies make more informed decisions. In addition, developing a library of standard questions allows for the reuse of questions in other studies and responses for future trials.
Evaluate how pre-study visits (PSVs) or qualification visits are done. Establish clear criteria around whether a PSV is required or can be waived to proceed with site selection. With the advancements in decentralized and digitally connected trials, remote PSVs are becoming more commonplace. This can provide cost and time benefits and accelerate site activation.
Sponsors and CROs can enable faster site engagement by establishing a site selection strategy focused on data, simplifying questionnaires, and clearly defining PSVs. Paired with modern study start-up applications, this approach can help the industry improve site engagement long-term and reduce the burden of using numerous systems for sites.
Tapping the Power of a Modern Study Start-Up System
A purpose-built solution can help sponsors and CROs bring together start-up activities and processes in one system. This includes building workflows, tracking and analyzing data, and leveraging automation to speed site engagement.
More importantly, sponsors and CROs can establish reusable data-driven exchanges with sites. An effective study start-up system should deliver a global directory of contacts, accounts, and site information; connected workflows, milestones, and documents that automate processes; reusable documents and data; and end-to-end reporting. With advanced capabilities, companies can eliminate wasteful manual steps from their site engagement strategy.
Using a single system to manage study start-up activities also establishes a strong data foundation, enabling real-time metrics and reports. This allows clinical leaders to prioritize and manage critical tasks and milestones across multiple studies. If issues can be identified and resolved quicker, teams can execute faster.
Here are key considerations for clinical leaders assessing study start-up solutions to advance their site engagement strategy:
- Alleviate the site burden. Sponsors and CROs should make every touchpoint with sites as seamless as possible. A one-stop shop study start-up system replaces spreadsheets, paper, and disparate tools while simplifying the site experience.
- Enable connected processes and workflows. Seamless information and document sharing between study start-up and other clinical applications, like clinical trial management systems and electronic trial master files, reduces administrative tasks for study coordinators and eliminates costly and complex integrations. The system should enable data flow based on sequential processes.
- Establish a site and investigator database. Gather and store clean, accurate data, including site performance statistics and facility information. Companies should gather information from site engagement to study completion for continuous use across all trials.
- Prioritize user experience. A user-friendly, role-based platform that provides a consistent user interface drives effective and consistent processes.
- Build a roadmap for the next five years. The path to streamlined study start-up and site engagement is a marathon, not a race—map details with clear goals, requirements, and expectations to drive continuous improvement.
- Evaluate trusted technology partners. Look for vendors with a proven track record of success. They should provide training and change management strategies and be equally invested in your success throughout the journey.
Enabling Long-Term Stakeholder Collaboration
Addressing the critical challenges during site selection and leveraging the power of modern systems can significantly improve how trials are run. Looking ahead, sponsors, CROs, sites, and patients should have one source to find each other easily. A platform that allows sponsors to search based on criteria, sites to share credentials and information, and patients to find trials will improve engagement and collaboration in trials.
Bringing stakeholders together to seamlessly share and access information can drive transformational change for the industry. If we can speed study start-up and clinical execution, life-changing medicines can reach patients faster.
References
- https://www.globaldata.com/increased-use-virtual-trials-contributed-improved-patient-accrual-rates-says-globaldata/
- https://www.fiercebiotech.com/biotech/new-research-from-tufts-center-for-study-of-drug-development-characterizes-effectiveness
- https://www.statnews.com/2019/08/23/clinical-trial-recruitment-diversity-community-engagement/

Anusha Shetty is Director of Clinical Strategy for Vault Study Startup at Veeva Systems.



