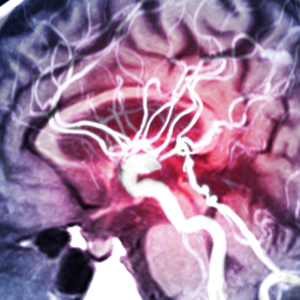
A study involving virtual rather than real patients was as effective as traditional clinical trials in evaluating a medical device used to treat brain aneurysms, according to new research. The […]
Data captured in one segment of the National Cardiovascular Data Registry (NCDR) suite of registries is similar in quality, depth, and granularity when compared to data captured through clinical trials, […]
Clinical trial practitioners should be aware of a new European Union (EU) Clinical Trials Regulation (Regulation No 536/2014) that will require lay (plain language) summaries for every clinical trial conducted […]
If necessity is the mother of invention, the COVID-19 pandemic parented a number of innovative leaps forward in the clinical trial industry. From the increased adoption of technology to the […]
When the U.S. Food and Drug Administration (FDA) calls to say it’s coming to inspect your study site, “it’s like a bomb drops, it’s total chaos,” says Neala Lane, MS, […]