Clinical Researcher—January 2020 (Volume 34, Issue 1)
SITE STRATEGIES
Bree Burks
As the age of blockbuster drugs has given way to precise, impactful treatments that are customized for rare diseases and individual patients, the volume of clinical trials has compounded. In 2007, there were 48,290 clinical trials recorded worldwide while more than 322,100 trials are expected to be tallied for 2019.{1}
With clinical studies flourishing, sponsors have shifted to an outsourcing model to drive greater efficiency. More than 70% of all clinical trials are expected to involve a contract research organization (CRO) in 2020,{2} and analysts predict the CRO market will reach nearly $55 billion by 2025.{3}
Clinical research sites are also becoming increasingly vital in helping sponsors and CROs keep pace with bringing new treatments to market. Sites, however, face significant hurdles in meeting the demands of this new era of drug innovation—hurdles that may discourage the all-important participation of principal investigators in studies. In fact, more than half of first-time investigators do not conduct a subsequent trial.{4}
Going forward, there is a significant opportunity for the industry to improve collaboration and execution of clinical trials. Sites have the most to gain, especially with improving productivity and reducing delays that impact trial execution. Recently, leading investigators from a range of different sites identified three key opportunities for improvement: communication and collaboration, information sharing, and streamlining common trial processes.
Improve Communication and Collaboration to Drive Productivity
Good communication often starts with mutual understanding. By getting to know one another and establishing common ground, clinical trial partners can be more productive and develop stronger working relationships. While the ability of sponsors and CROs to meet the needs of sites has progressed in recent years, most sites say there is room to improve communication,{5} especially around the day-to-day responsibilities and workloads that impact timelines at sites.
“Sponsors don’t have visibility into the many things on my plate to understand that their timelines are sometimes not achievable,” said Katie Seehusen, a regulatory specialist with the Iowa Diabetes & Endocrinology Research Center. “I am the only regulatory specialist at my site, and we currently have 30 ongoing drug and device studies with seven new studies coming soon. So, it’s important that I structure my time to get everything done.”
Seehusen frequently balances the critical deadlines of various sponsors and CROs, and ultimately has to make difficult judgement calls about which jobs get done first based on priorities and available resources. “I am pretty good at multitasking,” she said, “but sometimes there just isn’t enough time in the day. Just because we work at high volume, doesn’t mean we have a high volume of staff.”
The demand on sites to get more done, faster can be overwhelming. Sites acknowledge that better communication on timelines and even collaborating with sponsors and CROs on how to work more efficiently can alleviate some of the pressure they’re feeling.
Jeff Kingsley, CEO of IACT Health, a clinical research network based in Columbus, Ga., said part of the communication and collaboration breakdown often stems from the outsourcing model itself. “There’s sometimes a big gap between the sponsor and clinical trial sites with separate companies handling labs, oversight, and so forth,” he said. “If sites and sponsors become more strongly connected, we could better support the tremendous growth happening throughout the industry. Bridging the communication gap is critical to this.”
Streamline Information Exchange for Faster Trials
One of the most common needs among researchers is improving information exchange with sponsors and CROs.{6} Key drivers included reducing manual processes, improving collaboration among study partners, and better visibility and oversight (see Figure 1).
Figure 1: Top Drivers to Streamline Information Exchange
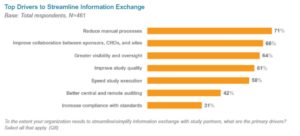
Sites often depend on sponsors and CROs to provide technology for managing trial activities and collaborating during a study. Sponsors and CROs on average use three different systems—including e-mail, portals, file sharing, and eTMF applications—to exchange trial data and documents with study partners.
Meanwhile, sites support multiple studies at one time. With each sponsor providing a unique system to sites running dozens or even hundreds of trials, the use of technology can become overwhelming. In fact, the average site uses a minimum of 12 different electronic systems to collect and capture clinical study data.{7}
“One of the biggest challenges we have as a site is that most technology comes from sponsors, and the sponsors select the technology that serves their needs,” said Rachel Sheppard, clinical research director for the Clinical Trials Units at the University of Louisville. “We’re running trials for hundreds of sponsors, so we have to learn all those various systems and be agile in them. So, it’s been difficult for us to transition to electronic regulatory systems because of that.”
Several sites noted that better information sharing could be addressed by newer cloud-based tools that are capable of streamlining trial activities and accelerating research.{8} Sheppard said that administrative minutiae are holding sites back and preventing trials from getting started quickly. “These setbacks could be resolved with better technology,” she added.
Nelson Rutrick, CEO of Adams Clinical, a site outside Boston specializing in psychiatric research, said sponsors collect large amounts of information on site data quality during patient enrollment. Making these data available helps sites understand where they’re doing well and where they need to improve.
“Sites know how well they are doing relative to other sites on recruitment metrics, but they often lack visibility into their performance on quality metrics,” Rutrick said. “Most electronic data capture and eSource products have reporting features that display some statistics to sites, but these features are usually turned off. Sponsors and CROs should turn these features on so sites can see where improvement is needed.”
Some sites also said making information sharing more efficient would enable them to focus more time and energy on other important matters—most notably training.
“I never want to be so busy that I stop learning,” said Seehusen. “Research is always changing. There are always new things to learn. We have a job where we constantly need to learn about what’s changing. I would love to have more time to train and be better at my job.”
Better training is an opportunity to improve clinical trials. Researchers want a roadmap for the right qualifications to do their job—the skills they should have and the related competencies. There’s a big movement among sites around competency-based roles. With so many specialized roles, the industry could benefit from sites having more ways to get credentialed for their unique roles.
Katrina Quidley, a regulatory manager with IACT, said she would like more sponsors and CROs to consider waiving redundant requirements for good clinical practice (GCP) training for staff with Certified Clinical Research Coordinator (CCRC) or Certified Principal Investigator (CPI) credentials through the Association of Clinical Research Professionals (ACRP). In 2014, the TransCelerate BioPharma Inc. initiative began acknowledging these ACRP certifications as an equivalent to any GCP course. Accepting ACRP certifications for GCP fulfillment streamlines the training process and allows sites to move forward with completing all personnel requirements and meeting expected timelines.
Simplify Operational Processes to Reduce Delays
With more study partners in the mix, trials have become increasingly complex. More people are involved in trial processes, each wanting to review and approve a site’s work. This often leads to trials being underbudgeted or delayed.
“There are many parties involved these days,” said Kingsley. “Innovation can stagnate because of so many in-betweens. CROs typically prefer that all communication be filtered through them, so we often lose the emotional connection with sponsors. Sponsors and sites don’t have much contact anymore.”
Seehusen said she often finds herself dealing with more people than she can count—all needing the same information. “Sponsors and CROs have a lot of people working on the trial, so there can often be a lot of redundant steps that cause delays,” she noted. “Centralizing coordination and improving organization could save me hours; and if you multiply that across all the studies happening at any given time, it could add up to days of improved productivity.”
Working Better Together to Improve Patients’ Lives
The pressure to innovate specialized products and get them to market quickly will only become harder as the ecosystem of clinical trial stakeholders continues to expand. Improving efficiency among study partners will enable the entire industry to better support the growing number of trials and, ultimately, bring innovative new therapies and drugs to market much faster for the patients who need them.
In our conversations with expert sources at sites, they underscored their desire to work with sponsors and CROs to improve trial processes. In the end, they said everyone just wants to achieve more positive results.
“Our biggest focus is on improving the lives of patients,” Seehusen said. “So, if we’re working at a capacity of maybe 15 studies a year and we can get to 20 studies a year because we’re working more efficiently and effectively, then we can see more patients and help more people. That’s really our end goal—just being able to help more people.”
References
- ClinicalTrials.gov. 2019. Number of Registered Studies Over Time. https://clinicaltrials.gov/ct2/resources/trends
- Business Wire. 2015. Research and Markets: The New 2015 Trends of Global Clinical Development Outsourcing Market. https://www.businesswire.com/news/home/20150130005621/en/Research-Markets-New-2015-Trends-Global-Clinical
- Grand View Research. 2019. Healthcare CRO Market Size Worth $54.7 Billion by 2025. https://www.grandviewresearch.com/press-release/global-healthcare-cro-market
- ScienceDirect. 2017. One and done: Reasons principal investigators conduct only one FDA-regulated drug trial. https://www.sciencedirect.com/science/article/pii/S245186541630093X
- CenterWatch. 2019. Sponsors, CROs Doing Better, Sites Say, But More Work Is Needed. https://www.centerwatch.com/articles/24465-sponsors-cros-doing-better-sites-say-but-more-work-is-needed
- Veeva Systems, Inc. 2019. Veeva 2019 Unified Clinical Operations Survey Report. https://www.veeva.com/resources/veeva-2019-unified-clinical-operations-survey-report/
- BioSpace. 2016. New CenterWatch Study Finds that E-Clinical Technologies are Increasing Investigative Site Work Burden and Performance Inefficiencies. https://www.biospace.com/article/releases/new-centerwatch-inc-study-finds-that-e-clinical-technologies-are-increasing-investigative-site-work-burden-and-performance-inefficiencies-/?keywords=centerwatch+study+finds+that+e-clinical
- Veeva Systems. 2019. Veeva announces free eRegulatory solution for Clinical Trial Sites. https://www.veeva.com/resources/veeva-announces-free-eregulatory-solution-for-clinical-research-sites/

Bree Burks (bree.burks@veeva.com) is Vice President, Site Strategy for Veeva Systems, with more than 12 years of experience in research in sponsor and site settings.



