Clinical Researcher—April 2020 (Volume 34, Issue 4)
SPECIAL FEATURE
Deaven A. Hough, MA; Elizabeth Flood-Grady, PhD, MS
Recruiting participants into research studies is one of the most difficult challenges we face as research professionals. The development of online tools, such as ResearchMatch and other participant-facing recruitment websites,{1} has enhanced research recruitment efforts. Study teams are also increasingly interested in using social media channels to bolster recruitment.{2}
At the University of Florida (UF), we’ve developed guidelines for recruitment on social media and launched a Facebook page, UF Studies, as a central channel for recruitment advertising and general information about study participation. We’ll give you a glimpse of these initiatives here, and we plan to present on this topic live through ACRP in the near future.
Creating Guidelines for UF Research-Study Teams
Despite the expansive reach of social media, there are generally limited directives regarding their use for study recruitment. In 2016, our institution identified the need for a coordinated approach to address privacy, information security, and other questions pertaining to institutional review board (IRB) submissions to enable researchers to use social media in an ethical and compliant way to recruit research participants.
Social media have generated a great deal of enthusiasm as recruitment tools, but simply planning to “post on social media” isn’t enough to effectively and ethically recruit participants. To harness the power of social media for study recruitment, UF’s Clinical and Translational Science Institute (CTSI) facilitated a committee and workgroup that endeavored to establish guidelines on how teams and institutions can ethically and effectively use social media channels for recruitment. Because multiple stakeholder groups are involved in the ethical recruitment of research participants and affected by social media recruitment decision-making, key stakeholders at the institution were involved in the development of guidelines from the beginning.
Dr. Elizabeth Flood-Grady presented a webinar featuring our process for identifying and engaging these stakeholders as part of the Trial Innovation Network (TIN) webinar series. You can watch the webinar here and download the slides here if desired.
Our guidelines emphasize:
- compliance with social media site terms of use;
- participant privacy, confidentiality, and data security; and
- procedures and considerations for using social media to recruit participants.
The guidelines focus on Facebook as the primary social media platform to recruit participants, due to the platform’s expansive reach and large base of users. Facebook, the leading social networking site worldwide, offers billions of users the unique opportunity to access and exchange health information,{3,4} including information about recruitment and participation in health research studies. Other social media channels are reviewed on a case-by-case basis.
We invite you to click here to read the full guidelines available on our website.
Facebook Advertising Through UF Studies
The UF CTSI’s Recruitment Center is funded by a Clinical and Translational Science Award from the National Center for Advancing Translational Sciences (NCATS), and serves as a central resource for study teams interested in recruitment assistance. The UF guidelines incorporate the establishment of a central UF Studies Facebook page, which the CTSI Recruitment Center manages and uses to advertise studies at the request of UF researchers and to disseminate other relevant information about research and research participation.
The CTSI Recruitment Center provides no-cost consultations for research teams identifying and evaluating study recruitment methods, including Facebook, and creating comprehensive recruitment strategies for individual studies and grants. We conduct a feasibility assessment for teams interested in recruiting through Facebook paid advertising or Facebook groups and pages. The vast majority of our Facebook recruiting efforts use paid advertising campaigns. We create Facebook recruitment plans for IRB approval, launch IRB-approved plans, monitor campaign progress, and track metrics on recruitment. Here, we’ll use a case study on recruiting adults for a Type 2 diabetes study to demonstrate this process.
Case Study: Diabetes
This randomized study’s population is age 21–75 with Type 2 diabetes in the Gainesville, Fla. area. Exclusionary criteria include a diagnosis of Type 1 diabetes, drinking three or more alcoholic beverages a day, or diagnosis of Hepatitis B or C.
To see if Facebook would be a good fit for this study, we conduct a feasibility analysis in the ads manager function on Facebook. The ads manager is also where we eventually launch and monitor the campaigns. First, we select the target audience that we want to see the ads. Target audience is determined by selecting the targeting criteria, including location, age, gender, demographics, and any potential interests that are relevant to the population who will see study advertisements.
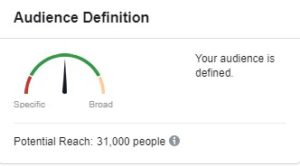
Click here to see Facebook’s infographic of all the areas teams can use to target participants. When we conduct a feasibility analysis, the goal is to have Facebook evaluate the audience as “defined,” and within the green section of the meter (as shown above).
Study teams cannot target prospective participants by health conditions. Instead, they can identify and target prospective participants by health condition–related interests.
For example, we cannot target individuals with Type 2 diabetes, but we can target by interests related to the “Diabetes Daily” or “Diabetes mellitus type 2 awareness” (as depicted below). We recommend turning to the Facebook Audience Insights tool to identify additional interests for your target audience.
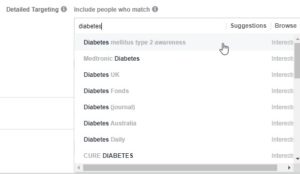
With general interests about diabetes, it is likely that individuals with Type 1 diabetes, an exclusion criterium for this study, may also see the ads. That is why developing targeted ad content, which is explained in detail below, is incredibly important.
The CTSI Recruitment Center creates a recruitment plan for the study team which contains:
- the list of targeting criteria (i.e., location, age, gender, demographics, and any potential interests);
- ad content, including a variety of post text, headlines, and images; and
- a description and link for where the ads will direct users.
The above considerations combine to make up a Facebook ad (see below).
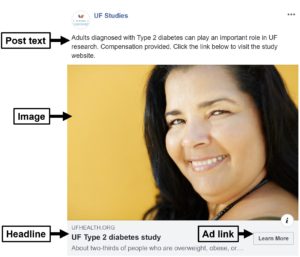
We create post text and headlines that are theoretically informed and based on previous successful Facebook advertising campaigns.
You can see in the ad we mention Type 2 diabetes in both the post text and the headline. Although adults with Type 1 diabetes may still see the ads, being as specific and targeted as possible in our ad content is an effective strategy for highlighting the relevance of the study to intended participants.
Images are selected from Shutterstock, as every Facebook ad manager has no-cost access to Shutterstock images.
The ad link should lead potential participants to more information about the study. Our Type 2 diabetes ad shown above links to a webpage with more information about the study. This webpage is what we refer to as a “study listing” that lives on our hospital’s website. The “study listing” is created by our CTSI Recruitment Center along with the Facebook plan with optimized content such as clear headlines and bulleted lists.
Linking the ads to study webpages with additional inclusion/exclusion criteria ensures the study team is not overwhelmed with requests from potential participants who would not qualify for study participation. In addition, by linking to a study listing on our hospital website, prospective participants see our health system logo and website URL and the study legitimacy is verified.{5,6}
Launching, Monitoring, and Tracking Campaigns
If the study team wishes to move forward with advertising its research on Facebook, we discuss the fees associated with using UF Studies and the CTSI Recruitment Center. Study teams are responsible for the cost of the campaign advertising and for CTSI Recruitment Center service fees associated with creating, launching, and monitoring the advertising campaign. The study team is also responsible for submitting all recruitment materials to the IRB for approval prior to launching a campaign on Facebook.
Once the study team has an IRB-approved plan, the CTSI Recruitment Center launches the advertising campaigns on the UF Studies Facebook page. Each campaign we launch on UF Studies allows us to learn more about recruiting participants using social platforms.
We won’t go into detail about the technicalities of launching the ads, but we do want to discuss monitoring Facebook ads. Let’s just say launching is the easy part.
Our guidelines address how to handle comments that reveal protected health information (PHI). For example, we had a Facebook user comment, “I am interested in the study, please call me at [phone number redacted]” and we hid the comment due to the reveal of the private information, even if it was self-disclosed.
The possibility of getting comments of this sort is very real, which is why having a plan in place to monitor, evaluate, and respond to comments is critical. Here at UF, we have a team that is available to help respond to or suggest deletion of comments.
In addition to monitoring comments, we track campaign metrics through Facebook’s ad manager function. This allows us to see how many clicks, people reached, and impressions the ad campaigns are receiving. We provide study teams with a weekly update on their campaign metrics and request data from them in return, including how many individuals contacted them because of the Facebook ads and how many participants they enrolled as a result.
Facebook Groups and Pages
Utilizing existing Facebook groups and pages is a good option for teams with a limited budget (it’s free) and if there is a specific disease condition where pages and support groups exist.
Although posting in groups and pages is free, there is no guarantee that the Facebook algorithm will display the post to the intended audience. Paid advertising allows us to track metrics such as clicks, reach, impressions, and budget. Posting to groups and pages does not allow for those analytics unless specifically requested from the group or page moderator.
For our Type 2 diabetes study, we searched for groups and pages that are relevant to the study population in the local area.
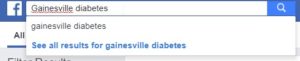
When searching for groups, we want to make sure we are being as specific as possible. Posting to bigger groups might at first seem like a good way to reach large numbers of people, but can result in queries and questions that are difficult to manage due to the sheer volume of the audience you are reaching. Study teams must submit a social media management plan for these posts to the IRB for approval; these plans must address not only how inquiries will be handled, but also how PHI posting will be addressed.
We do not recommend study teams join private groups. Instead, we suggest that the study team asks the Facebook page administrator to post on behalf of the study team. This not only protects the potential participants, but also relieves study teams from having to monitor comments and questions.
Results and Lessons Learned
So, does Facebook advertising work? We think so; since launching our UF Studies page in 2017, we have seen incredible growth in our page and paid advertising results (as of March 2020):
- 39 campaigns launched
- 39,000+ link clicks
- 868,000+ people reached
- 2,000,000+ impressions
Most importantly, nearly 1,800 participants have enrolled into studies at UF directly from paid UF Studies Facebook campaigns. Behavioral studies, particularly those requiring completion of an online survey, make up a large proportion of studies at UF. While some of our clinical trials are tough to recruit for and may not have any enrollments from Facebook, we still see a lot of engagement such as comments, likes, and shares from our ads.
As we move into 2020, we hope to understand a bit more about engagement on Facebook and just what sort of role it plays in recruitment. To date, our results demonstrate that Facebook advertising has incredible potential to enroll research participants, though a common concern is the ethics of this recruitment method.
When someone signs up for a social media platform, such as Facebook, they agree to the terms of use, but it is our responsibility to ensure that any use we make of the platform takes the rights and welfare of potential participants into full consideration. This is why we highly recommend institutions that are interested in using social media to recruit research participants take the time to understand the roles and responsibilities of different institutional offices (e.g., the IRB will review social media content) and research teams (e.g., teams must adhere to existing social media policies enforced at the site). This also includes the permissible channels, strategies, and mandatory information to include in a social media management plan.
Perhaps one of the biggest lessons we have learned is that there isn’t just one way to use social media—or even Facebook specifically—to recruit research participants. While other platforms have generated enthusiasm, we are continuing to evaluate our progress with Facebook groups, pages, and (of course) paid advertising. Having established relationships with your IRB, site communications team, and overall leadership is essential, as Dr. Flood-Grady describes in her TIN webinar.
While Facebook shows promise, running paid ads or posting in groups and pages is not an automatic “win” for recruitment. When a study team comes to the UF CTSI Recruitment Center and expresses interest in using Facebook, we recommend a wide variety of services through our consultations and feasibility analysis. Indeed, we often suggest that study teams combine Facebook advertising with other services, such as ResearchMatch, our hospital’s research registry, and our community engagement program, HealthStreet.
The UF CTSI offers a wide variety of resources to study teams across the university, specifically research services like our CTSI Recruitment Center. To strengthen our services, we work closely with the STEM Center for Translational Communication at the UF College of Journalism and Communications. Researchers and communications professionals from both groups work together to improve human health by incubating health communication research across disciplines and making scientific research more accessible, understandable, and usable.
As we continue to launch, monitor, and evaluate Facebook as a recruitment tool for research studies, we invite you to share your own social media recruitment experiences with us. We would love to collaborate with you and exchange information to ensure that study teams across the nation are using effective recruitment tools that are tailored to research participants.
References
- Flood-Grady E, Paige SR, Karimipour N, Harris PA, Cottler LB, Krieger JL. 2017. A content analysis of Clinical and Translational Science Award (CTSA) strategies for communicating about clinical research participation online. J Clin Transl Sci 1(6):340–51. doi:10.1017/cts.2018.2
- Topolovec-Vranic J, Natarajan K. 2016. The use of social media in recruitment for medical research studies: a scoping review. J Med Internet Res 18(11). doi:10.2196/jmir.5698
- Strekalova YA, Krieger JL. 2017. A picture really is worth a thousand words: public engagement with the National Cancer Institute on social media. J Cancer Ed 32(1):155–7. doi:10.1007/s13187-015-0901-5
- Strekalova YA, Krieger JL. 2017. Beyond words: amplification of cancer risk communication on social media. J Health Comm 22(10):849–57. doi:10.1080/10810730.2017.1367336
- Gulliver A, Griffiths KM, Christensen H. 2010. Perceived barriers and facilitators to mental health help-seeking in young people: a systematic review. BMC Psychiatry 10(1):113. doi:10.1186/1471-244X-10-113
- Sawyer DC, Lambert D. 2006. Rural and frontier mental and behavioral health care: barriers, effective policy strategies, best practices. National Association of Rural Mental Health (Waite Park, Minn.). doi:10.1037/e546002013-001

Deaven A. Hough, MA, is a Recruitment Specialist with the University of Florida Clinical and Translational Science Institute.

Elizabeth Flood-Grady, PhD, MS, is a Postdoctoral Associate at the University of Florida specializing in translational health communication, mixed methods, and dissemination.



