Clinical Researcher—May 2020 (Volume 34, Issue 5)
CRA PATHWAYS
Jason Methia, MS
With clinical study monitors and patients restricted from travel, sites, sponsors, and contract research organizations (CROs) are reassessing ways to keep existing trials on track while also initiating and expediting new trials related to COVID-19. Analysis of federal clinical trials data found that the pandemic has forced thousands of trials to slow, stalling critical research into drug therapies and other interventions.{1}
The pandemic is also accelerating the industry’s efforts to change how trials are monitored now and in the future. Even so, clinical trial monitoring has been ripe for change for several years. The shift away from traditional onsite monitoring practices has been supported by regulators and industry stakeholders for years.
In 2013, the U.S. Food and Drug Administration (FDA) recommended the shift to remote monitoring to improve data quality, increase efficiency, and reduce costs of clinical trials.{2} Earlier this year, with the emergence of the novel coronavirus, the FDA further encouraged the move, stating that, “If planned onsite monitoring visits are no longer possible, sponsors should consider optimizing use of central and remote monitoring programs to maintain oversight of clinical sites.”{3}
Purpose-built systems designed to support remote monitoring can deliver needed improvements in data quality, efficiency, and overall costs of trials—along with several benefits to sponsors, CROs, and sites.
Improve Data Quality
Faced with a significant amount of paper documentation for a clinical trial, clinical research associates CRAs must manually verify data contained within hundreds of files for source data review (SDR) and source data verification (SDV), which can lead to review inconsistencies.
In an attempt to share information with monitors remotely, sites often shift to printing, sharing documents via e-mail or enabling direct access to the electronic medical records (EMRs). Unfortunately, these methods are complex and, when done manually, have the potential to introduce errors or compliance issues with the Health Insurance Portability and Accountability Act (HIPAA). While these systems and processes may provide an immediate solution, they do not improve data quality or reduce compliance risk in the long term.
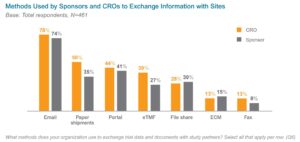
E-mail and paper methods of exchanging information across sites, sponsors, and CROs also add to the levels of data-sharing and collaboration complexity. In a 2019 survey, 96% of sponsor and CRO respondents said they have significant challenges with the methods used to exchange information during clinical trials (see Figure 1 for more information). These difficulties can contribute to issues respondents have with tracking and reporting (71%) and with misfiled or missing documents (57%).{4}
Figure 1
Purpose-built remote trial monitoring solutions, which are commonly built into the site’s eRegulatory or electronic investigator site file (eISF), enable CRAs to review documents and conduct oversight activities without ever setting foot in the physical site. With direct access to regulatory and source content, CRAs can spot problems and trends more easily to improve quality. In addition, higher level metrics, such as safety issues or turnaround times, can be more easily identified. More specifically, the system capabilities should include:
- Access controls—Remote monitoring systems can streamline sharing of source information to authorized individuals to prevent unauthorized access to identifiable patient information and reduce tracking and filing errors.
- Audit trails—All actions taken on a document are time stamped and recorded to ensure data integrity and improve accountability.
- Reporting—Site- and study-level trends, such as safety issues or protocol deviations, can be more easily tracked to prioritize reviews and identify potential compliance concerns.
Increase Efficiency
Since 90% of study-specific source forms are still created on paper,{5} sites and CRAs spend a large portion of their time collecting and organizing files, as well as tracking detailed communications via e-mails, attachments, and phone calls.
Remote monitoring systems can track the detailed, back-and-forth monitoring conversations in a central location and can automatically alert site coordinators and CRAs when feedback is needed, reducing effort and improving turnaround times. Onboarding new staff is quicker and easier when systems are standardized and repeatable workflows guide them through reviews. Plus, the ability to view historical communications and actions via audit trails helps new members reconstruct the history of a trial more quickly.
Remote monitoring technology can also promote efficiency in the following ways:
- Repeatable workflows—With step-by-step workflows, sites can add CRAs directly into the review process. Monitors then review each document and provide their review status before completing the next step in the workflow, ensuring nothing gets missed. Additionally, historical comments from previous reviews can be maintained, ensuring effort is not accidentally spent reviewing the same document twice.
- Organize and prioritize work—Documents and tasks can be automatically flagged based on their type, status, and due date to help CRAs and coordinators quickly focus their efforts and plan their day-to-day work.
- Support collaboration—Alerts can notify sites and CRAs when new documents are ready for review. Upon review, monitors can flag specific text or phrases in question and route documents back to the site for clarification. When feedback is needed, the CRA can quickly locate the document or phrase in question and respond in a timely manner.
- Improve standardization—By reducing process variation and the number of different technologies used for monitoring, sites and CRAs can become more proficient in the systems. Using fewer technologies also means less time spent switching between applications, thus improving CRA efficiency and study quality.
Reduce Costs
While launching and running a clinical trial is expensive overall, onsite monitoring alone comprises 25% to 30% of total clinical trial costs.{3} Most of the expenses stem from the travel costs of CRAs: transportation, hotel, and meals add up quickly, especially when issues or complications require the CRA to physically travel to the site multiple times during a trial or on short notice.
Remote monitoring systems reduce the overall costs of launching and running a clinical trial, which in turn means sponsors are able to run a greater number of concurrent trials. In addition, the time savings realized with remote trial monitors helps speed trial timelines, thereby bringing needed therapies to more patients, sooner. More specifically, remote monitoring reduces trial costs in the following ways:
- Requires less travel—With fewer onsite visits, monitors spend less time traveling and more time on reviewing data and supporting sites.
- Conserves site resources—Site staff can save time and conserve resources, such as office space and computer equipment, that would otherwise be needed to support onsite activities.
- Reduces turnover—Eliminating the need to travel onsite can alleviate burnout and help reduce CRA turnover.
Bring Critical Therapies to Market Faster
As the benefits of remote monitoring systems become increasingly clear, the ultimate goal of enabling sponsors, CROs, and sites to operate on a single, unified platform will further streamline clinical data collection and monitoring processes, thereby speeding clinical trials.
Ultimately, reducing trial timelines is an industry imperative. Whether conducting a trial for a new cancer therapy or a COVID-19 vaccine, valuable research must be allowed to continue in this “new normal,” and to be conducted faster while keeping participants safe.
By untethering oversight activities from physical sites, critically important research can continue and even accelerate. Systems that support remote monitoring can speed and simplify the data collection, oversight, and reporting processes that can keep trials on track during these difficult times. Most importantly, they can accelerate the development and approval of drugs and vaccines.
As the industry moves toward virtual clinical trial solutions, participants, monitors, sites, and sponsors alike will benefit. Remote trial monitoring plays a huge role in this transformation and in the future of clinical trials.
References
- National Public Radio. 2020. Coronavirus Pandemic Brings Hundreds of U.S. Clinical Trials to a Halt. (link)
- FDA.gov. 2013. Oversight of Clinical Investigations—A Risk-Based Approach to Monitoring. (link)
- FDA.gov. 2020. Coronavirus (COVID-19) Update: FDA Issues Guidance for Conducting Clinical Trials. (link)
- Veeva Systems Inc. 2019. Veeva Unified Clinical Operations Survey Report. (link)
- Society for Clinical Research Sites, 2017. Why is Clinical Source Data Still Collected on Paper? (link)

Jason Methia, MS, focuses on Site Engagement and eTMF Strategy for Veeva Systems. Prior to joining Veeva in 2013, he held a variety of roles within clinical development at the Dana Farber Cancer Institute, Wyeth Research, and Vertex Pharmaceuticals.



