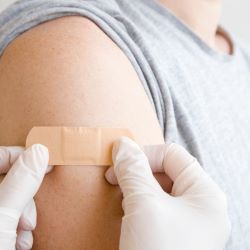
“Drug developers recognize the importance of participant demographic representation in the development of safe and effective medical treatments,” said Ken Getz, professor and deputy director of the Tufts Center for […]
As the clinical trial industry staggers through an epic pandemic called COVID-19, there’s been precious little time to think about planning for 2021 and beyond. However, with some states now […]
Clinical Researcher—May 2020 (Volume 34, Issue 5) PEER REVIEWED Seema Garg, MS, MBA, CQA, CSSGB In light of the current COVID-19 crisis, we are seeing an intense focus on […]
Clinical Researcher—May 2020 (Volume 34, Issue 5) SPECIAL FEATURE Daniel Eisenman, PhD, RBP, SM(NRCM), CBSP The global pandemic caused by the coronavirus (SARS-CoV-2) and the disease it causes (COVID-19) […]
Clinical Researcher—May 2020 (Volume 34, Issue 5) CRA PATHWAYS Jason Methia, MS With clinical study monitors and patients restricted from travel, sites, sponsors, and contract research organizations (CROs) are […]