Clinical Researcher—May 2020 (Volume 34, Issue 5)
SPECIAL FEATURE
Daniel Eisenman, PhD, RBP, SM(NRCM), CBSP
The global pandemic caused by the coronavirus (SARS-CoV-2) and the disease it causes (COVID-19) have brought biosafety to the forefront of most everyone’s minds. Things like hand hygiene, personal protective equipment (PPE), and respiratory protection are no longer niche topics.
In the research community, we’re seeing a surge of interest in the requisite safety practices for research involving specimens from SARS-CoV-2 positive individuals and COVID-19 subjects. The Centers for Disease Control and Prevention (CDC), the Occupational Safety and Health Administration (OSHA), and the American Biological Safety Association International (ABSA International) have issued guidelines for implementation of well-established safety practices for such research.{1–4}
Start with a Risk Assessment
According to the CDC’s Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19), “[a]ll laboratories should perform a site-specific and activity-specific risk assessment to identify and mitigate risks.”{1}
The CDC’s emphasis on “site-specific” and “activity-specific” assessments is telling—there is no single approach that ideally fits all laboratories and all research procedures. Each risk mitigation plan must be based on the potential biological hazards unique to the activity and the environment where the activity takes place. The CDC notes the assessment and accompanying risk mitigation measures depend on:
- The procedures performed
- The hazards involved in the process/procedures
- The competency level of personnel performing the procedures
- The facility and its laboratory equipment
- The resources available
Risk assessments begin with defining the risks associated with the hazard. SARS-CoV-2 is believed to be transmitted through exposure of the mucous membrane such as the eyes, nose, and mouth with:
- Infectious respiratory droplets, and/or
- Direct contact with infected body fluids, and/or
- Exposure to contaminated fomites (such as contaminated PPE or used tissue paper).{5}
The virus may also be transmitted by inhaling infectious aerosols.{6,7} The virus’ genetic material (RNA) has been detected in various types of clinical specimens including blood, urine, feces, anal swabs and oropharyngeal swabs, as well as sputum and bronchoalveolar lavage fluid.{8,9} Respiratory secretions are associated with higher levels of viral RNA and viral transmission and are therefore considered to pose the highest risk of the specimens mentioned. The presence of SARS-CoV-2 RNA in patient blood implies infection via exposure to contaminated sharps is a possibility; however, the likelihood is unknown. With the hazards identified, the next step is discerning the level of risks posed by different experimental procedures.
Developing the Risk Mitigation Plan
A comprehensive risk mitigation plan will address foreseeable risks associated with the research materials, starting with their origination and transport to the laboratory and ending with waste treatment and disposal. The risk mitigation plan should also address incident response and reporting. Comprehensive risk mitigation plans must also include procedures to follow in the event of spills or exposures to infectious agents. A thorough risk mitigation plan will address:
- Employee training regarding the hazards
- Necessary safety precautions for employees to safely conduct their duties
- Engineering controls and PPE necessary to safely conduct the research
- Treatment and disposal of infectious/regulated medical waste
- Occupational health and incident response
- Administrative controls such as inspections and competency drills
The OSHA general duty clause states employers must provide employees a work environment free from recognized hazards that are likely to cause serious physical harm or death.{10} Work involving human blood and tissues is subject to the OSHA bloodborne pathogen (BBP) standard.{11} The BBP standard requires employers to determine which employees are at risk of an occupational exposure to bloodborne pathogens and ensure they are properly trained, provided with PPE, enrolled in an occupational health program, and offered hepatitis B vaccinations.
OSHA also requires employers develop a bloodborne pathogen exposure control plan (ECP). Depending on the type of proposed research, the ECP may also serve as a risk mitigation plan. At the very least, the ECP or an existing biosafety manual may provide a starting point for drafting a risk mitigation plan for coronavirus research.
Here are some considerations for developing the risk mitigation plan:
Low-Risk Procedures
Low-risk procedures which are not anticipated to produce infectious droplets or aerosols may be performed in a Biosafety Level 2 (BSL-2) laboratory as long as standard precautions are taken when handling clinical specimens. This includes proper hand washing practices and the use of appropriate PPE, such as gloves, lab coats or gowns, and eye protection.
Procedures that are not anticipated to produce infectious droplets or aerosols include receiving potentially infectious specimens and performing microscope-based assessment of fixed slides. If working with unfixed samples, the use of surgical masks, N95 respirators, or biosafety cabinets may be recommended, based on a risk assessment.
Procedures with Potential to Generate Droplets or Aerosols
Procedures with mild to moderate potential to produce infectious droplets or aerosols can be performed in BSL-2 laboratories with enhanced safety practices. Such procedures should be performed in an adjoining room from the rest of the laboratory. The room should have a door which can be shut to restrict access, and occupancy should be limited to the fewest number of individuals necessary to perform the laboratory procedures. Inward airflow should be exhausted directly out of the lab without recirculation to the rest of the facility.
All procedures with potential to produce infectious droplets or aerosols should be performed within a certified biosafety cabinet (see Figure 1) or other HEPA filtered aerosol containment device. Respiratory protection such as N95 respirators or powered air purifying respirators should be considered, based on a risk assessment. Centrifugation should be performed within centrifuges equipped with safety cups or sealed rotors loaded and opened within a biosafety cabinet. If utilizing suction to aspirate infectious liquids, the procedures should be performed within a biosafety cabinet, and the vacuum line should be equipped with a HEPA filter.
Figure 1: Biosafety Cabinet

Biosafety cabinets provide an enclosure to contain droplets and operate under negative air pressure (i.e., suck inward) to protect users from exposure to aerosolized infectious agents. Any infectious aerosols are sucked in through the grills positioned at the front and back of the work surface and removed by HEPA filtration. The HEPA-filtered exhaust protects the community and environment from exposure to aerosolized research materials. The hood also blows sterile, HEPA-filtered air from the top toward the work surface to protect the research materials from contamination with non-sterile, ambient room air.
Virus Isolation, Characterization, and Vaccine Studies in Animal Models
While a typical BSL-2 laboratory provides adequate containment for diagnostic assays as well as quantification of viral and antibody titers, Biosafety Level 3 (BSL-3) containment is required for culturing and characterizing the virus or conducting vaccine challenge studies in animal models. BSL-3 laboratories are designed to contain aerosol transmissible pathogens (see Figure 2).
Figure 2: Key Features of BSL-2 and BSL-3 Laboratories
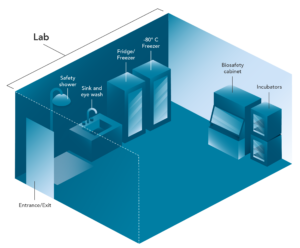
Biosafety Level 2 (BSL-2) laboratories are designed for work with clinical specimens and microorganisms that cause mild to moderate disease in healthy adults. Lockable doors restrict access and allow posting of hazard signage. A sink with an eye wash is available as well as a safety shower.
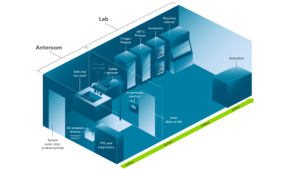
BSL-3 laboratories are designed to contain aerosol-transmissible pathogens. Design criteria builds on requirements for BSL-2, including an anteroom; visual airflow indicators; unidirectional, inward-flowing, single-pass air that should be exhausted through a HEPA filter; biosafety cabinet(s); and an autoclave.
BSL-3 lab design criteria include a two-door entry system to provide security and an anteroom for donning and doffing PPE (including respiratory protection). The laboratory must have unidirectional, inward-flowing, single-pass air that should be exhausted through a HEPA filter. Visual airflow indicators such as air pressure monitors must be available. All work must be performed within a negative-pressured and HEPA-filtered biosafety cabinet or another aerosol containment device. The facility must contain an autoclave for sterilizing infectious waste.
The Most Common Laboratory Procedures for COVID-19 Research
The two most common procedures conducted on COVID-19 specimens are the real-time polymerase chain reaction (real-time PCR or RT-PCR) and the enzyme-linked immunosorbent assay (ELISA).{12} RT-PCR is a genetic test used to confirm the presence of the viral genome. The technology can also be applied to measure viral load.{13,14} ELISAs are utilized to quantify the amount of a specific protein in a sample. That protein may be the viral spike protein used to confirm infection or the research participant’s antibody proteins against the spike protein. The latter can be used to determine if an individual has been exposed to the virus and how well his or her immune system is mounting an adaptive immune response; this is an essential measure for vaccine efficacy studies.
Obtaining the final readout for these tests involves low-risk procedures. However, the following initial steps in setting up these assays run the risk of creating infectious droplets or aerosols, which creates an occupational exposure risk for lab staff:
- Extracting the viral RNA for RT-PCR
- Pipetting participant samples into an ELISA plate, as well as the aspiration and washing of these plates
Transporting Specimens from COVID-19 Patients
Potentially infectious materials are contained within a primary container, such as a blood collection tube, and should be transported within a facility in a durable, leak-proof, and biohazard-labelled secondary container, such as a sealed specimen bag. Shipping or transport of packages containing infectious materials requires a triple-packaging system.
Outside an individual facility, regulations for transport and shipment of infectious substances are provided by the U.S. Department of Transportation (DOT) for ground and International Air Transport Association (IATA) for air.{15–17} Both DOT and IATA require training for individuals who package hazardous materials as well as for those transporting or shipping hazardous materials.
The training requirement for individuals preparing the package means research participants cannot ship their own specimens without DOT/IATA-compliant training. Personnel transporting specimens must also have DOT/IATA-compliant training. It is worth noting the “Exempt Human Specimen” classification only applies to specimens for which there is a minimal likelihood that pathogens are present, based on professional judgement of the source’s known medical history, symptoms, and endemic local conditions. The exemption does not apply to specimens which contain or are suspected of containing infectious agents.{17} Specimens submitted to the CDC for SARS-CoV-2 testing must be shipped as Biological Substance Category B, rather than Exempt Human Specimens.{18}
Work Surface Disinfection, Waste Treatment, and Disposal
Equipment and work surfaces utilized in conjunction with infectious agents should be disinfected at each step in the experimental process and after any spills. Simply wiping down a surface with soap and water will not neutralize these agents. Instead, utilize an Environmental Protection Agency (EPA)-registered disinfectant that is labeled to be effective against SARS-CoV-2, and follow the manufacturer’s recommendations on dilution, contact time, and safe handling. The EPA provides a list of disinfectants with established efficacy against SARS-CoV-2.{19}
Treatment and disposal of infectious waste/regulated medical waste vary by state, so check your local regulations for specific guidance. This may involve contacting your organization’s environmental health and safety department, your institutional biosafety committee (IBC), or the state health department or EPA.
Decontamination of Spills
When spills of infectious materials occur, the focus must be on protecting research personnel as well as containing and disinfecting the spill. Personnel in the area must be instructed to leave and allow droplets to settle and aerosols to be removed by the ventilation system. Exposed personnel can utilize this time to treat any wounds or exposures as well as replace contaminated PPE.
Once an adequate period has lapsed, employees may re-enter the spill area and place disinfectant-soaked paper towels around the spill, moving from the periphery toward the center of the spill. The manufacturer’s recommended contact time must be allowed for adequate disinfection before disposing of the waste as infectious waste.
Post-Exposure Procedures
The risk mitigation plan must address post-exposure procedures to be followed immediately after the exposure, such as washing the exposed site or use of an eye wash, as well as seeking medical evaluation. Exposed employees may be asked to remain in isolation at home and self-monitor for signs and symptoms of coronavirus infection for the duration of the 14-day incubation period. The occupational health provider may require daily reports in the form of calls or e-mails, which may include body temperature readings and listing any signs or symptoms of infection.
Incident Reporting
The principal investigator/supervisor must be notified of any incidents in the laboratory. Furthermore, various types of reporting may be required, based on the research oversight structure at a site as well as local health department requirements. Exposures must be reported as part of the organization’s exposure control plan and documented in the OSHA 300 incident log.
Also, IBC-approved research must be reported to the Biosafety Officer/IBC. National Institutes of Health (NIH) Guidelines require immediate reporting to the NIH Office of Science Policy in the event of spills or accidents resulting in an overt exposure to engineered genetic materials in laboratories operating at Biosafety Levels 2 or greater.{20} Local requirements may also mandate reporting to the local health department.
Expert Review of Risk Mitigation Plan
It is good practice to have the risk mitigation plan reviewed by a panel of subject matter experts to ensure it comprehensively addresses the risks associated with the proposed research. A hospital’s bloodborne pathogen committee may seem like the appropriate panel to review the plan, but such committees typically focus on standard of care and medical procedures involving sharps rather than review of laboratory-based research protocols. Assessing risks involving recombinant DNA and infectious agents may be outside the committee’s scope or realm of expertise.
An IBC may be a better fit for reviewing the risk mitigation plan, as IBCs focus on risk assessment and risk mitigation for research involving engineered genetic materials and may cover microbiological safety. Institutions that have conducted NIH-funded research and conduct research involving engineered genetic materials are required to have IBCs.{20} Sites without IBCs may opt to have their risk mitigation plans reviewed by an independent commercial IBC or another third party. Having a documented plan reviewed and approved by an independent panel of subject matter experts provides the highest level of safety and legal protection to everyone involved.
Depending on local requirements, an organization’s risk mitigation plan may need to be filed with the local health department.
Conclusion
The initial risk assessment is key to determining the appropriate risk mitigation measures for coronavirus research at your location. Biosafety practices must be customized to the proposed research as well as the existing facility and local regulatory requirements. Proper biosafety measures are critical to conducting safe, high quality research during this uncertain time.
References
- Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention.
- Worker Protections Against Occupational Exposure to Infectious Diseases. Occupational Safety and Health Administration.
- Considerations for Handling Potential SARS-CoV-2 Samples. American Biological Safety Association International.
- Biosafety in Microbiological and Biomedical Laboratories. National Institutes of Health and the Centers for Disease Control and Prevention.
- How COVID-19 Spreads. Centers for Disease Control and Prevention.
- Droplets and Aerosols in the Transmission of SARS-CoV-2. Meselson M. 2020. NEJM.
- Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. van Doremalen N, et al. 2020. NEJM.
- SARS‐CoV‐2 can be detected in urine, blood, anal swabs and oropharyngeal swabs specimens. Peng L, et al. 2020. J Med Virology.
- Detection of SARS-CoV-2 in Different Types of Clinical Specimens. Wang W, et al. 2020. JAMA.
- Occupational Safety and Health Act of 1970. 29 USC 654, section 5(a)1
- Blood Borne Pathogen Standard. Occupational Safety and Health Administration. 29 CFR 1910.1030 in the Code of Federal Regulations.
- CDC Tests for Covid-19. Centers for Disease Control and Prevention.
- Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Yu F, et al. 2020. Clinical Infectious Diseases 35(6).
- Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: The Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. Lim J, et al. 2020. J Korean Med Sci.
- Hazardous Materials Regulations. Department of Transportation (49 CFR Parts 171–180 in the Code of Federal Regulations).
- Transporting Infectious Substance Overview. Department of Transportation, Pipeline and Hazardous Materials Administration.
- Dangerous Goods Regulations. International Air Transport Association. (3.6.2.2.2 and 3.6.2.2.3)
- Guidelines for Submitting Specimens to CDC for Laboratory Testing For SARS-CoV-2. (Version 1.2) 2020. Centers for Disease Control and Prevention.
- Disinfectants for Use Against SARS-CoV-2. Environmental Protection Agency.
- NIH Guidelines for Research Involving Recombinant or Synthetic Acid Molecules. National Institutes of Health, Office of Science Policy.

Daniel Eisenman, PhD, RBP, SM(NRCM), CBSP, (daniel.eisenman@advarra.com) is Director of Biosafety Services for Advarra in Research Triangle Park, N.C.
The author would like to acknowledge the assistance of Stephanie Pyle, Shaun Debold, Sarah Bowman, and Katie Krull in the preparation of this manuscript.



