Clinical Researcher—November 2020 (Volume 34, Issue 9)
SPECIAL FEATURE
Anna Argyris, MA, CCRP
It is an ongoing problem for the clinical research enterprise that the ever-growing layers of complexity involved in conducting clinical trials make it harder for sites to work efficiently and collaborate with sponsors and contract research organizations (CROs) across their studies. Research teams struggle to balance an increasing number of information requests from sponsors while providing high-quality care to study participants. Stakeholders in the industry are moving quickly to find better ways of working together, but the overreliance on paper-based processes makes study execution difficult.
Many sites today still capture information using paper, spreadsheets, or fragmented point solutions that weren’t built for their operational needs. Research administrators use manual, time-consuming processes to fill in the blanks between information silos and systems that don’t talk to each other.
To reduce administrative burden, investigative sites are shifting toward more digital, connected ways of working by adopting site-centric technology. These advanced solutions can standardize site operations, streamline information exchange, and improve research staff engagement to accelerate clinical research and deliver critical treatments to patients faster.
Standardize Processes Across Studies to Enable Paperless Trials
Solutions specifically built for sites help investigators standardize processes across studies to drive greater efficiency, improve compliance, and run paperless trials. Regulatory document management, for example, is a key area for modernization.
An eRegulatory system can replace paper binders and standardize how documents are stored and managed, increasing efficiency. With an eRegulatory system that can handle electronic signatures, document version control, certified copy workflows, and full text document search, sites can reduce the burden on research teams to scan and manually upload files to shared drives and across multiple sponsor portals.
Sites can also enable self-service access to regulatory documents for sponsors, freeing up research teams to focus on patients. Over time, sites can connect their eRegulatory solution to sponsor systems to simplify information exchange while ensuring secure, controlled access to their study information. Research sites are now rightfully accelerating the shift toward modern, site-centric solutions to streamline regulatory processes.
Site-centric solutions should include standard document naming conventions to identify content quickly, automatic document filing to eliminate the need of manually filing a document across multiple studies, and security roles and access levels to ensure the right people can get to the information they need (see Figure 1). These features simplify processes and maintain compliance, improving how trials are run.
Figure 1:
Standardizing site operations enables sites to measure quality and compliance across research and frees up study teams to focus on providing care and treating patients. It also can significantly improve how sponsors, CROs, and sites work together during trials, enabling a more transparent, paperless, and efficient way of managing studies.
Streamline Information Sharing Among CROs, Sponsors, and Sites
Sites generate a significant amount of paper documentation during a clinical trial that is incredibly difficult to share with sponsors and CROs. The original source, which is typically paper, combined with printing electronic medical records (EMRs) and wet-ink signatures, often leads to manual and paper-based information exchange.
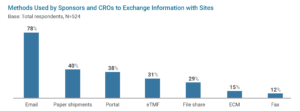
According to the 2020 Unified Clinical Operations Survey Report,{1} e-mail (78%), paper shipments (40%), and portals (38%) were still found to be the predominant way sponsors and CROs exchange information with sites (see Figure 2). Unfortunately, these methods increase administrative effort, reduce efficiency, and lengthen the clinical trial process. Nearly all respondents report significant challenges with the methods used to exchange information with sites.
Figure 2:
Many sites still print records for their study monitors, adding complexity to information sharing in trials and keeping the site reliant on paper. When onsite monitor visits stopped during COVID-19, many sites shifted to sharing documents via e-mail or fax or enabling direct access to their EMR system.
Adopting site-centric technology can resolve many of the existing issues with information exchange in clinical trials. One of the key enablers for better collaboration between sponsors and sites is a modern remote monitoring solution.
When sponsors and CROs have different study monitoring requirements, it can increase administrative work and process disparity for sites. By standardizing on one remote monitoring solution, sponsors, CROs, and sites can reduce time-consuming onsite visits, improve oversight, and remove logistical barriers that can slow study timelines.
Looking toward the future, remote monitoring will be an essential part—even a requirement—for clinical trials going forward. While remote monitoring provides clear benefits to sponsors and CROs by reducing travel costs and increasing utilization, research sites can easily standardize monitoring efforts to achieve efficiency gains that speed execution.
Site-centric solutions that enable investigators to standardize across studies ensure consistency, reduce training fatigue, and improve trial data quality. Sites can then spend more time with patients and less time preparing administrative requests. The result is streamlined information sharing across end-to-end processes for better collaboration and, ultimately, faster trials.
Improve Proficiency and Reduce Training Burden
Research teams express frustration from working with multiple disparate systems with different processes, logins, and training requirements.{2} To enable people to work smarter, sites are adopting site-centric solutions.
When systems are built to streamline and standardize site operations across studies, the burden of training is lowered. Trading multiple point solutions and manual processes for a single system that handles investigator site files and remote monitoring provides value by making it easier for staff to become more proficient.
Site-centric solutions reduce logistical challenges such as managing multiple logins and losing system access that can slow trials. Fewer systems and logins lead to fewer questions and access issues for study teams, allowing more time for high-value tasks like delivering care to patients. Having one site-owned system ensures that documents are safe and filed consistently across studies, despite turnover of research coordinators and clinical research associates.
Site leaders can also gain insights across trials that help them make better, more informed decisions. For example, if users can see how long it takes to collect signatures, they can focus efforts on improving turnaround times—and if they can see when documents are about to expire, they can do a better job on updates to improve compliance and operate more efficiently.
Creating a Go-Forward Plan
Investigators looking to build a resilient research infrastructure can start by evaluating software created specifically for sites by a technology partner who understands investigator pain points. Consider providers who are focused on site success, who are well known and trusted by sponsors and CROs, and who advocate for site needs. Taking these criteria into account helps speed the acceptance of new systems and positions sites for long-term success.
Once the site’s leaders have decided to implement a site-centric solution, it is time to put together a plan. The following steps will help guide sites toward success:
- Know what success looks like. Evaluating new technology requires clear goals and outcomes, such as improved visibility, consistency, quality, and speed. Identify the key performance metrics that will be used to determine if these goals have been met.
- Identify pain points and barriers to success. Evaluate processes that are manual, time-consuming, error-prone, or not designed for your needs. For example, if unplanned downtime, paper-based processes, or variance across departments and studies are slowing trials down, determine more sustainable ways of working.
- Focus on outcomes, not features. When evaluating systems, start by determining the optimal outcomes and rank them in order of importance. With clear objectives in mind, research teams are less likely to be tempted by every “bell and whistle” a system can offer, and can stay truly focused on the functionality that addresses the challenges the site is looking to overcome.
- Evaluate the software provider, not just the software. When evaluating vendors, look beyond their sales and messaging to understand their history, reputation, approach to building software, and proven customer success. Consider the vendor’s ownership profile, financial stability, and development methods, and then speak with other customers about these factors. Consider a proven technology provider with a track record of innovation and success with sites and acceptance across the industry.
Conclusion
Sites that standardize operations, improve collaboration and information exchange, and make it easier for research teams to become proficient and streamline study execution will be positioning themselves for success in the clinical research enterprise going forward. With site-centric solutions, investigators can achieve higher levels of standardization, efficiency, quality, and stakeholder engagement. If sites can run trials faster and more efficiently, the industry can accelerate the delivery of new drugs and treatments to the patients who need them.
References
- Veeva Systems. 2020. Unified Clinical Operations Survey Report
- XCRS. 2019. Impact Assessment of eClinical Technologies and Industry Initiatives on Sites

Anna Argyris, MA, CCRP, (anna.argyris@veeva.com) is Director, Site Solutions at Veeva Systems. She has more than 10 years of clinical research leadership experience and has worked at Mayo Clinic, Georgetown University, Houston Methodist, and The University of California, San Francisco. Her expertise includes study feasibility and logistics planning, finance and forecasting, program development, coverage analysis, billing compliance, and business process design.



