Clinical Researcher—April 2021 (Volume 35, Issue 3)
PEER REVIEWED
Holly Bookless, BSN, RN, NE-BC; Paula Smailes, DNP, RN, CCRP; Todd Lusch, BA; Deanna Golden-Kreutz, PhD
What was originally known as the extramural General Clinical Research Center (GCRC) program was funded from the 1960s, first by the National Center for Research Resources within the National Institutes of Health (NIH), and then as part of the Clinical and Translational Science Awards (CTSA) program via NIH’s National Center for Advancing Translational Sciences up until about seven years ago.{1} While the NIH-backed funding dynamic has changed over the years and the GCRC itself has been defunded, NIH CTSA support to academic medical centers (AMCs) has had a lasting impact. With or without current CTSA funding, integral programs at AMCs across the United States continue to yield successful research thanks to infrastructure created with federal help for facilitating studies that may be otherwise challenging for investigators to complete.
The Clinical Research Center facility (hereafter referred to as “the center” in most cases) at The Ohio State University Wexner Medical Center (OSUWMC) is now based within the College of Medicine’s Center for Clinical Research Management and includes 11 beds, a metabolic kitchen, a processing lab for biological samples, and an analytical and development lab for assays.{2} The center’s staff consists of a nurse manager, a fiscal officer, two clinical research coordinators, six nurses, a registered dietitian, two clinical research assistants, and three research lab technologists. These resources currently support 130 active protocols and involve more than 2,500 participant visits per year. Studies span multiple therapeutic areas in Phases I through IV for both inpatient and outpatient settings. While the predominant hours are 7 a.m. to 5 p.m. Monday through Friday, 24/7 care can be provided when necessary for inpatient participant studies.
COVID-19 Impact on Business Operations
While the normal, busy operations of the center were occurring a year ago, the threat of COVID-19 came to fruition when the Governor of Ohio announced on March 12, 2020 that the state would be shutting down many operations to help prevent the spread of COVID-19. With that, research leadership began determining which research studies would continue versus those that should temporarily stop. It was decided that only therapeutic trials would be continued. While most studies did well shutting down, concern did exist for some investigators with respect to study and funding timelines.
The Clinical Research Center at OSUWMC, with which all the authors of this article are engaged, remained open and continued research activity. Because of this, we were able to help with studies that did not otherwise have available research staff.
Alternative Work Arrangements
With a reduction in workloads, initiatives were created to keep staff off campus when possible. Some center staff transitioned to work from home and needed assistance with resources, which included setting up equipment and network access. Guidance from research leaders was provided for what activities would be appropriate to do from home. When applicable, staff were assigned work that did not involve direct participant care, such as data entry, standard operating procedure development, equipment ordering/inventory, and professional development.
One particular concern related to the possibility of COVID-19 exposure for the center’s staff and participants, along with the possibility of transmission. Therefore, staff were also assigned to develop safety workflows to mitigate COVID-19 risk when working at the center.
Supporting the Health System
Space
As with most AMCs, space is a hot commodity at OSUWMC. As a result, the medical center conducted an assessment of space to determine what areas could be used for a possible rise in COVID-19 admitted patients, including use of the Clinical Research Center. With a possible upsurge of COVID-19-positive patients, leadership determined that the center could possibly house COVID-19-positive patients or formerly positive patients awaiting a second negative swab.
The building that houses the center also includes the medical center’s rehabilitation hospital. The rehabilitation hospital began to have space issues related to the need to keep patients in private rooms for proper distancing; for this reason, patients were moved into open beds located in the center. Research supplies and equipment had to be moved and stored in office spaces and other rooms to accommodate the rehabilitation patients moving in temporarily. Rehabilitation patients without COVID-19 were sent to the center and occupied nine beds. Since rehabilitation nursing is a specialty area, the associated nursing staff followed these patients to care for them on our unit.
The center housed the rehabilitation patients from the end of April until July 2020. In mid-July, the center returned to normal operations; however, in mid-August the rehabilitation patients returned due to space issues. This time, the move was simplified by sharing some supplies, while keeping important billable and research supplies separated. As a result, “research only” space for staff and research equipment was created. The ultimate configuration of the center during this time and currently is that COVID-19-positive participants are on one end of the unit (three beds), COVID-19-negative research participants on the opposite end (three beds), and medical/surgical patients from University Hospital in the middle (six beds, with three being semi-private). Specialty equipment for the center had to be moved out and is being stored until normal operations resume.
Staff
While the center’s operations were being revised, a plan for redeployment of staff to other areas of the medical center began in response to the potential increase in hospital occupancy due to inpatient and critically ill COVID-19 patients. Five of the six existing nursing staff were identified for potential deployment to assist with a potential COVID-19 surge. In preparation, these nurses underwent specialized training for the electronic medical record and general patient care; this included possible deployment to intensive care units, depending on nursing experience. To date, there has been no need to deploy center staff to other units. Hospital and research leadership determined that research staff would be one of the last groups to be deployed due to their critical role with forwarding the research mission, including the conduct of important therapeutic COVID-19 studies at our AMC.
Conducting COVID-19 Research
When the majority of the world was closed due to the emerging pandemic, the Clinical Research Center needed to continue supporting the medical center’s activity of direct patient care through research while also developing processes to manage the impact of the COVID-19 virus. Initially, the center began working on several studies related to exposures to COVID-19 and frontline healthcare workers. Overall, the center completed close to 1,300 visits with healthcare workers from May to July 2020. This experience prepared the team to move forward quickly when the College of Medicine’s Office of Research approved a restart of both non-COVID-19 and COVID-19-positive studies in July 2020.
The first study was for those with mild to moderate COVID-19 symptoms for an outpatient monoclonal antibody study. This study required participants to be onsite at the center. At the same time, COVID-19 vaccination research was preparing to start. This research required the center to complete visits if the participant became ill after being vaccinated.
Challenges and Solutions
These studies necessitated consideration regarding the logistics of COVID-19-positive and COVID-19 high-risk participant movement and samples. A COVID-19 Clinical Care Committee reviewed the research protocols allowing COVID-19-positive participant studies at the center. As part of the review process, an epidemiologist walked through the unit with the study team, principal investigator, and the center staff. Questions arose about processes that needed to be developed, including the following:
- What building entrance should be used for COVID-19-positive participants?
- Where should personal protective equipment (PPE) be sourced in order to not interfere with clinical needs for PPE?
- How do we complete procedures and handle samples safely with COVID-19-positive participants?
- How do we lessen our staff’s exposure and decrease their overall time at the bedside with COVID-19-positive participants?
- How can we lessen the risk for COVID-19-negative inpatients and non-COVID-19 research participants on the unit while completing COVID-19-positive studies?
- How will the rooms be cleaned after COVID-19-positive participants leave?
To answer the questions about these challenges, solutions were created (see Table 1). To execute COVID-19-positive studies, the study sponsor or sponsoring department purchased PPE and other necessary supplies. N100 masks and replacement filters were supplied by our organization. Special training for proper donning and doffing of PPE was reviewed with staff as provided by the medical center. Hand hygiene was stressed, with hand sanitizer dispensers being located in all patient rooms and outside all patient doors. Institutional hand hygiene policy requires nurses to clean hands upon entry and again when leaving patient rooms, after touching any surfaces, and prior to and after gloving and/or any patient contact.
Table 1: Challenges and Solutions During the Pandemic
| Challenge | Solution(s) |
| Shortage obtaining supplies/PPE | · Ask sponsor to provide first, then organization. |
| COVID-19 patient logistics | · Consult epidemiology for staff workflows and participant activity.
· Develop guidance for unit activity and participant safety. |
| Staff working from home
|
· Assign remote duties such as data entry, policy development, and computer-based learnings for professional development.
· Provide computers and monitors and ensure connectivity. |
| Study teams and center staff fears and concerns for COVID-19 transmission | · Provide emotional support.
· Provide education related to safe COVID-19 practices. · Refer to employee assistance program for mental health services. |
| COVID-19-positive patient exposure time
|
· Communicate through the Updox system and conduct virtual participant monitoring.
· Conduct continuous assessment of processes and support to adjust procedures quickly as needed. |
| Research participant safety and prevention of COVID-19 transmission
|
· Cluster COVID-19-positive participants away from non-COVID-19-positive participants.
· Terminally clean rooms after use. · Use PPE and established hand hygiene practices. |
Collaboration to reopen research activities was done with the following support areas:
- The Clinical Research Center Registered Dietitian worked with the Respiratory Therapy and Epidemiology Departments to understand metabolic cart equipment operation and cleaning after use for COVID-19-positive participants, which is designed to perform indirect calorimetry, oxygen consumption, and exhaled CO2
- Epidemiology was consulted for ongoing review of procedures to ensure processes were safe.
- Respiratory therapists were involved in spirometry and exercise studies so that their input could be gained into the safe operation and cleaning of equipment during the pandemic.
COVID-19-Positive Study Participants in the Center
COVID-19-positive study participants were screened prior to arrival at the center to ensure eligibility. If during the recruitment process it was determined that a participant did not qualify, they did not come to the unit and were advised to follow up with their primary care physician. However, upon arrival eligibility was again reviewed; participants could potentially be screen failures once at the unit due to unstable vital signs. For COVID-19-positive participants coming to the center, a workflow was developed to ensure safety for staff and patients (see Figure 1).
Figure 1: COVID-19-Positive Participant Workflow in the Clinical Research Center
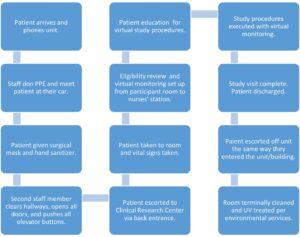
COVID-19-Positive Participant Arrival
After the COVID-19-positive participant parks their car, the participant calls the center nurses’ station to notify staff of their arrival and the assigned number of their specific parking spot. The participant would remain in their car while CRC nurses donned the proper PPE including N100 mask, hair cover, face shield, gown, and gloves. The nurses would meet the participant at their car with hand sanitizer, a surgical face mask for the participant to wear, and an ID band to identify the participant.
The participant was not permitted to touch anything, including the door, elevator button, etc. In some cases, they would be taken by wheelchair to the unit. Participants and staff would enter the back side of the unit away from the nurses’ station and most other unit activity. Another staff member would help with opening doors, using their badge for entry, and clearing the hallway of patients and staff.
There was a commitment to having strong communication with other staff working in the building, so they were aware of what was occurring. Height and weight were collected on the way to the room on a designated scale. These participants had mild to moderate symptoms and only a few participants had any shortness of breath or cough.
Study Execution
Once the research participant was in a center room, vital signs, pulse oximetry, and a general medical/surgical history were collected. Virtual monitoring was done with the utilization of Updox to reduce staff exposure time with the COVID-19-positive participant. (Updox is a Health Insurance Portability and Accountability Act–compliant, web-based communications platform using video to interact with patients virtually.)
A study doctor would perform the physical and ensure participant eligibility before nurses started their research care. Once the participant qualified, the center nurse worked with the participant to do urine pregnancy testing (if applicable), IV placement for blood work, vital signs, and review of surgical, medical history, current medications, and allergies. The nurse also completed a nasal swab and saliva sampling for the COVID-19-positive diagnosis and, most importantly, ensured the participant is comfortable.
For ongoing monitoring, a laptop was placed in participant’s room and a second laptop was placed at a desk near the telemetry monitoring system. The link to Updox is sent to the nurse caring for participant. The center staff member opens the link on the laptop in the room and ensures video and sound are enabled. Another staff member or nurse stationed near the telemetry machine ensured the participant was visible, including the ability to see chest rises for respiration monitoring, face, and IV medication pump reading.
Staff donned and doffed PPE several times depending on study activities per medical center policy. Virtual monitoring helped to reduce the amount of PPE used and lessened staff exposure, thus promoting safety.
For studies with investigational IV medication administration, the nurse caring for the participant does as many activities as possible pre-infusion/medication administration. The nurse also ensures a good visual is in place from the desk via the laptop in the participant’s room. The participant is instructed on how and when to take their temperature when blood pressure monitoring is completed. Pulse oximetry measurement is checked continuously and this can be seen virtually at the nurse station.
A staff member located at the center’s nurse station checks respirations, heart rate, and blood pressure every 15 minutes and continuously monitors the participant during infusions to ensure there are no issues with tolerance and side effects. The nurse caring for the participant must re-enter the room when investigational medication is ready per pharmacy and leaves the room so virtual monitoring can be done at the desk.
COVID-19 Study Samples
Samples are sent to the analytical and development lab for processing within the Biosafety Cabinet For nasal swabs, once the specimen is in the medium, it is believed to no longer pose a threat of exposure to staff; however, it is still handled per lab safety standards and required PPE. The swab is placed in a biohazard bag and is wiped with a disinfecting wipe. A second biohazard bag is placed over the first biohazard bag and is also wiped. Glove changes and hand washing occur before and after each bag is applied. Swabs are delivered to the lab and they are labelled as COVID-19-positive, packaged, frozen, and shipped.
Patient Discharge
Per consult with epidemiology, Sani-wipes® are used by the nurses upon discharge to effectively kill COVID-19 on surfaces and provided by the institution for this purpose. After COVID-19-positive patients are discharged, the room is terminally cleaned and UV-treated per environmental services and institutional policy.
Support for Non-COVID-19 Studies
COVID-19 research was being conducted at the same time as non-COVID-19 studies. Staff members were given a rotation of work assignments (concentrating on non-COVID-19-positive assignments on some days and focus on COVID-19-positive assignments on others) and highlighting and celebrating when goals such as a top recruiting site and/or the first site to enroll a participant were achieved.
Discussions occurred with individual research teams and lead center staff regarding the safety and plans to minimize exposure to COVID-19 for participants and research staff. The center’s nurse manager oversaw these discussions while the staff nurses would review the revised center processes and provide insight on institutional rules and policies.
Study teams were asked to do COVID-19 pre-screening on all participants. Participants were met outside due to the locked doors of the center building. Participants were required to wear surgical masks provided by the study team. Masks were essential and had to be worn by the participant for the entire research visit despite the length of the study visit. Some visits could last for 10 to 12 hours, and education was provided to the participant prior to study visits regarding the need to wear a mask the entire visit.
The New Normal
As we continue to work toward routine research operations, the COVID-19 pandemic promises to bring more barriers to the conduct of clinical research. However, overcoming challenges and capitalizing on the solutions we’ve developed will hopefully allow the Clinical Research Center to be better prepared in the future. At the same time, potential new options for the growth of research operations exist with tele-research visits, electronic and virtual consenting, and other virtual research activities.
Many future opportunities to study and learn about COVID-19 are inevitable. The processes and procedures the center has in place, including support from research leaders, essential open communication, participant safety, and teamwork among staff, will sustain the operations of our center for both COVID-19-positive and COVID-19-negative studies. This promises to promote the research enterprise at our organization and beyond.
References
- Nathan DG, Nathan DM. 2016. Eulogy of a Clinical Research Center. J Clin Invest 126(7):2388–91. doi:10.1172/JCI88381. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4922700/#:~:text=The%20first%20extramural%20GCRC%20grants,and%20therapeutics%20in%20volunteer%20patients
- https://ccts.osu.edu/content/clinical-research-support
Holly Bookless, BSN, RN, NE-BC, (holly.bookless@osumc.edu) is the Nurse Manager for the Clinical Research Center/College of Medicine’s Center for Clinical Research Management at The Ohio State University Wexner Medical Center.
Paula Smailes, DNP, RN, CCRP, (paula.smailes@osumc.edu) is a Visiting Professor at Chamberlain College of Nursing and Senior Systems Consultant at The Ohio State University Wexner Medical Center.
Todd Lusch, BA, (todd.lusch@osumc.edu) is Director of Clinical Research Operations and Implementation for the College of Medicine’s Center for Clinical Research Management at The Ohio State University Wexner Medical Center.
Deanna Golden-Kreutz, PhD, (deanna.golden-kreutz@osumc.edu) is the Senior Director for the College of Medicine’s Center for Clinical Research Management and Associate Clinical Professor for the Department of Internal Medicine at The Ohio State University Wexner Medical Center.



