Clinical Researcher—April 2021 (Volume 35, Issue 3)
PEER REVIEWED
Christina Morris; Erika Stevens, MA
Biotechnology, medical device, and pharmaceutical companies are seeking other industries’ disruptive digital technologies to aid in improving operational efficiencies and resourcing. For example, customer-centric robotic process automation (RPA) is thriving as an approach to handling routine financial processes in information technology, warehouse management, customer service, and healthcare areas,{1} and its wider applications have attracted interest from more research-oriented sectors.
Think about your all-in-one banking app; through the user interface, the customer is delivered the content or data they need, when needed. The user interface and the desired end-user (customer) experience (outcome of the interaction) are usually orchestrated by a digital process application that is integrating and/or connecting with other applications or data sources. The data orchestration manages the data, and the user interface manages the end-users’ desired experiences (see Figure 1).
Figure 1: Conceptualizing the User Experience, User Interface, and Customer Experience
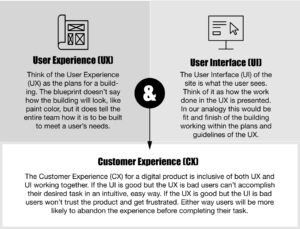
In other words, you tap or click on weblinks or buttons, the application leads you through a further series of sequenced, process-driven taps or clicks, and voila! You now have a new account and an e-mail notification to alert you of the new account and account activity. In reality, the e-mail system is not a part of the banking account system, so data and/or process orchestration software combine to coordinate the process, exchange data, and create e-mail notifications. Even with all the complicated processes going on “behind the scenes” as financial legacy systems are integrated, you are delivered a seamless customer experience resulting in user-friendly applications now at your disposal.
You may be wondering how these concepts apply to the clinical research enterprise. Similar to the evolution of other industries, the drug/device/biotechnology sector’s reliance on research professionals, both internally for preclinical development of potential new products and through contract research organizations and study sites for testing of the same in humans, grew organically to meet the demand of the global market under constant regulatory scrutiny, as well as inorganically to capture segments of the market it wanted to capitalize. Over time, more functions and departments were created and organized to complete the tasks that required specific knowledge (or what was deemed specific knowledge).
The additive result of organic and inorganic growth is that each newly organized function or department within the sponsors of research, their subcontractors for managing studies, and the sites at which the research is actually conducted seemingly required its own software to solve its own problems without the users fully evaluating what they were trying to solve in cooperation with each other.
Today, we have multiple functions conducting slightly different tasks with much of the same data in hundreds, if not thousands, of different software systems and are living in a very big plate of people, process, systems, and data spaghetti.
This approach has increased technical debt over the years, leaving drug developers, for example, with about the same timeline of 12 to 15 years to bring a new product to market, increased costs to go to market, and an absolute necessity for corporate digital strategy leadership.{2}
How do we get from the proverbial people, process, systems, and data spaghetti mess to something that is strategic, reusable, and future-enabled? Where do we even begin? In practice, we observe life science executives—under pressure to show progress in digitizing their assigned functional areas—screening their activity universe for digital innovation potential and launching a portfolio of (often disconnected) functional initiatives. However, this approach will only continue to make more spaghetti.
A strategic and data-centric approach is needed to implement and master the true power of digital technology. Digital strategy (combined with business and information technology [IT]) is required to fundamentally redefine the purpose of corporate IT/business missions across functional boundaries, along the entire value chain, and offers deep strategic, data, process, and operational insights.
SIDEBAR: The Terminology
Before we dive into technology, process management, and the overall IT landscape, it is helpful to understand the quality management system (QMS), process, and IT vernacular we are using. These terms are merely a guide to better understanding why we identify a problem and then apply a fit-for-purpose solution.
Digital enablement: Digital enablement is process of establishing business and IT digital strategies to achieve an intelligent, efficient, and effective business model. Digital enablement focuses on delivering an extraordinary customer experience (CX).
Disruptive digital technologies: Innovative software, applications, and/or technology that significantly change the course of daily activity and/or displaces an established tool.
Legacy systems/applications: Legacy systems are established IT applications. They are generally functionally focused, such as a clinical trial management system (CTMS) or electronic data capture (EDC) system. They can be a commercial off-the-shelve system or custom built and require integrations for the movement of data from one system to another. They do not focus on a process, but rather they are built as database structures/architecture with a frontend user interface/user experience (see below).
Swivel chair process: Swivel chair process is the activity of manually entering the same data in several systems within a process or series of processes. The end-user is effectively swiveling in and out of systems to complete a task.
User interface (UI): The user interface is the actual screens, buttons, and fields (we call them wire frames). The number of clicks, the location of the buttons, the information that is collected, the number of screens to collect, the scroll function, etc. are all functions of the UI.
User experience (UX): The user experience is the overall personal, internal experiences one has with an application. The collection of these experiences determines how end-users interact with the application and their unique experiences. UX works in concert with UI.
White space process: White space is the people, process, or systems gap between process steps or process handovers. For example, a CTMS manages information on the investigator running a trial but does not manage the processes that are required for a successful study launch.
SIDEBAR: Manual vs. Automation
Process data are often not “reused,” but instead “recreated,” requiring end-users to manually access several systems throughout their day to retrieve, manipulate, and manage the data they need to complete their activities.
Manual pain points: “Extra time” is required to download, review, and connect data through spreadsheets or other applications, send data to colleagues, and/or upload onto SharePoint/collaborative applications.
Automation application: Wraps around workarounds and “white spaces” between legacy systems where automation is typically deployed. This is a great place to utilize a disruptive technology alone or in combination with other automation technologies.
Automation impact: Activities are identified upstream and “connected” and/or reused as required. In the downstream environment, activities or processes focusing on the overall process and individual activities or silos that currently exist in most companies are addressed through data reuse.
The Quality Management System
We see the QMS at the epicenter of digital disruption in life sciences. Its unique organizational position allows the QMS to enable and facilitate a truly connected, end-to-end perspective on data, process, risks, and opportunities along a company’s value chain.
To tackle the issues already outlined, we will outline a data-strategic, process-focused, user-centric, and technology-enabled use case for QMS. This disruptive approach is designed to enable better connectivity between activities and functions, and to create an environment of future-proofing which is an industry-sustaining approach as well as a reproducible operational model enabling an offensive, constantly improving, and agile workplace.
The ultimate goal is to create an experience that keeps end-users engaged, is easy to use, and motivates compliant behavior—all while avoiding inefficiencies, such as having to access and transfer data between several systems during daily activities. Among the emerging technologies, digital process automation (DPA), the aforementioned robotic process automation (RPA), and blockchain are currently being “lifted and scaled” into drug and device research and development operations (front and back offices) to integrate/connect the data and end-user (see Table 1).
Table 1: Pros, Cons, and Process/Areas of Impact of Disruptive Digital Technologies
| Digital Technology | Pros | Cons | Process/Areas of Impact |
| Robotic Process Automation (RPA): Point application that leverages computer software to capture, interpret, and integrate data with existing application(s) for processing a transaction, data manipulation, and cross functional communication. The technology is similar to a macro. | – Increased accuracy
– Increased consistency – Audit trail – Increased productivity – Increased elasticity/flexibility – Cost effective – Implementation: relatively easy and quick |
– Point solution
– May take several automated software applications for one process – Does not “fix” process (bad processes remain bad) – Limited to simple tasks – Not enabled for artificial intelligence (AI)
|
– Logging into safety application to confirm submission
– Monitoring a clinical trial budget for changes and updating system with change – Moving investigator address from clinical trial management system (CTMS) to enterprise resource planning (ERP) system |
| Business/Digital Process Automation (DPA): Managing data and information across processes and legacy IT systems. This type of automation is built on process and activities with multiple business rules to guide end-users through their workflows resulting in the reduction of swivel chair time, additional point solution costs, resource burn, and offshore investment. | – Process-centric view
– Holistic view – Cost reduction – Increased accuracy – Increased connectivity – Audit trail – Increased productivity – Increased elasticity/flexibility – Reliability – Rules-driven |
– Implementation: Generally working across several functions and may be lengthy
– Multiple integrations – Requires process mapping – Requires understanding of resourcing – Requires understanding of local business/compliance/regulatory rules
|
– Integrating with CTMS, EDC, and enterprise financial software to orchestrate the payment process for investigators and clinical trial vendors
– Submitting safety reports from regulatory rules (from regulatory information management systems) |
| Cognitive Intelligence: The ability of computer software and the system to perform tasks autonomously that typically require human intelligence. Examples include visual perception, speech recognition, decision making, and language translation/interpretation. This type of technology enables the end-user to “check” and or monitor the aforementioned examples and eliminate the administrative task of manually entering, translating, or scanning multiple materials to determine a cause/correlation. | – Collates and automates decision making
– Audit trail – Increased productivity – Increased elasticity/flexibility – Reliability – Holistic view – Increased accuracy – Educated next best action |
– Implementation: More difficult than RPA and other forms of automation
– Multiple integrations – Requires process mapping – Requires regulatory scrutiny – Requires understanding of local business/compliance/regulatory rules |
– Managing selection of principal investigators
– Digital management of the entire safety process, including initial causality, triage, and signal detection – Managing quality assurance risk and audits |
| Blockchain: Distributed ledger of information/data that stores a history of all transactions that have occurred within the ledger in a linear/chronological order. Blockchain enables safe and secure transfer of private information within the ledger. | – Increased data quality and accuracy
– Increased data access – Increased revenue – Increased data security – Decreased cost |
– Newer technology
– Still under limited application in research and development
|
– Investigator qualifications
– Operational and back office financial process (over the counter, inventory management, etc.) |
The overall IT and business strategies connect business processes, leverage robust master datasets, improve efficiency, and increase transparency/regulatory compliance. By implementing proven technologies and software, companies can leverage existing lessons learned from other industries to increase production, regulate compliance, develop novel programs faster, increase employee satisfaction, and improve customer feedback.
Despite its recent interest in disruptive digital technologies, the life science industry was slow to adopt newer digital process automation tools to develop a holistic, risk-based approach to managing data/operations. Further, the industry mainly focuses on external regulators as the key customers causing the life science digital toolbox to remain innovatively stagnant and relatively immature compared to other industries. This immaturity is creating significant opportunities for improvement in operations, resource allocation, and systems integration. Examples are:
- Process white space around clusters of documented regulated activities
- Multiple software point solutions
- Multiple workarounds
- Underdeveloped risk management frameworks
- Lack of process/system integration
Disruptive Technologies
Data are the Currency of the Digital QMS
By connecting process data through the use of several synergistic digital technologies, we are now able to manage the process and data across the value chain.
We determined that the following functions and processes would benefit most from digital enablement:
- Preclinical
- Quality
-
- Audit management
- Risk management
- Clinical operation/Early development
-
- Study start-up
- Contracts/Third-party vendor management
- Clinical supplies management
- Pharmacovigilance/Drug safety
-
- Contracts/Third-party vendor management
- Regulatory affairs
-
- Regulatory application process
- Contracts/Third-party vendor management
- Medical affairs
-
- Contracts/Third-party vendor management
- Call center management
The Cost Impact
Multiple forms of automation can be used to assess the people, process, and IT landscape and enable the right technology at the right place at the right time. Further support of automation as the digital currency of the future comes from The Institute for Robotic Process Automation, which has seen a cost reduction of 35% to 45% for onshore operations and 10% to 30% for offshore operations. Gartner has claimed that 30% to 40% of existing business process services are likely to be impacted by automation.{3}
The Benefit/Risk Balance
Today’s automation technologies reduce white space processes between legacy systems by securely connecting and or interfacing with historically hard to interface legacy systems. Generally, automation mimics human action and eliminates the need for the end-user to connect multiple systems to complete one’s activities or tasks and/or focus on value-added, critical activities.
By utilizing and combining the aforementioned technologies, automating the business processes enables the employee to move through and work around the white spaces while the system pushes the next activity to the employee. Instead of manually beginning the next activity, the employee is automatically prompted. This process automation can achieve the following for any business:
- Reduce direct and indirect costs
- Improve accuracy, consistency, and reliability
- Process complex, high-volume activities more efficiently
- Improve process controls and risk-based approaches
- Increase agility and improve operational management offensive
- Integrate workflows for a more seamless approach
- Connect data sources
- Reduce cost of quality
- Offer more transparency for better decision making
Not all technology is created equal and there are inherent risks in selecting and implementing automation technology. What are the key risks and potential issues for enabling a fully automated QMS?
- Complexity: Due to the rapid growth and evolution in technology, it may be difficult to assess if the right technology has been selected or if there is a better less expensive technology in the pipeline.
- Scalability: Another question to consider is whether the tool or technology is scalable and fit for purpose. In some cases, the technology is fit for purpose and does not need to scale up or down to connect to other upstream or downstream processes. However, in some cases, the technology/tool will need to scale up to adjust for global requirements. Proper assessment of the tool would define the risks of fit for purpose and scalability.
- Change: Finally, how will the technology keep up with the change in regulatory agency modernization and the potential change in standards? Simply put, today’s automation needs to be prepared to meet tomorrow’s regulatory expectations. Again, this analysis is accomplished by conducting a proper assessment of the technology and its history to perform, update, and integrate over time.
How does the industry continue to facilitate change and influence automation technology? First, finding and adapting mature software that is being used successfully in other industries is key.
Imagine sharing data to enable better patient safety data or clinical outcomes, or sharing concepts and lessons learned in working groups or open research forums. It’s all possible with the right technology that ensures data are entered once and reused, not recreated.
The ultimate success when introducing a new drug/device/biotechnology product is defined by production results in the least amount of time and cost possible. All the while, the product must be safe, efficacious, and compliant with regulatory demands. These standards lead to multiple functions and tasks within a manufacturing process that must be managed in increasing tedious detail.
No matter how a new pharmaceutical product is produced, the end result must deliver an experience that keeps end-users engaged, is easy to use, motivates compliant behavior, and avoids inefficiencies, such as having to access and transfer data between several systems during daily activities. Among the emerging technologies, DPA, RPA, and blockchain are currently being “lifted and scaled” into clinical research enterprise operations (front and back offices) to integrate/connect the data and end-user together. The overall IT and business strategies are to enable connected business processes, leverage robust master datasets, improve efficiency, and increase transparency/regulatory compliance.
Conclusion
Digital strategy provides capabilities to move information through process automation. Leveraging existing data systems and tying them into an automated process reduces manual document creation.
The goal of digital strategy transformation drives a rethinking of an organization’s service model delivery. Coupled with streamlining processes across existing silos and improving efficiency, directed actions or decisions drive business functionality. Improved digital interaction capabilities enable increased production and drive experience. As a result, customer satisfaction is improved through transparency.
Aligning with a QMS, a disruptive approach designed to enable connectivity, sustainability, and continuous evolution fosters agility in the workplace. Fixing the foundation to implement a digital strategy fundamentally redefines the business mission, improves customer experiences, and improves the bottom line.
References
- https://www.linkedin.com/pulse/robotic-process-automation-revolution-business-technology-patel/?articleId=6675755793763049472
- https://www.protiviti.com/sites/default/files/united_states/insights/board-perspectives-risk-oversight-technical-debt-limiting-competitiveness-issue109_protiviti.pdf
- https://www.gartner.com/en/newsroom/press-releases/2019-10-02-gartner-says-robotic-process-automation-can-save-fina

Christina Morris is Vice President for Digital Transformation with Hoverstate, and has worked as a Clinical Research Associate and in clinical operations, quality assurance, and process improvements for a variety of organizations. She has been designing and implementing digital process automation applications across the life science value chain for more than seven years.

Erika Stevens, MA, is the 2021 Chair of the Association Board of Trustees for ACRP and leads Transformation Advisory Solutions for Recherche Transformation Rapide.



