Clinical Researcher—May 2021 (Volume 35, Issue 4)
TRIALS & TECHNOLOGY
Jim Reilly
Clinical transformation accelerated during COVID-19 to speed trial execution. As companies raced to find treatments and vaccines, life sciences researchers came together to deliver innovation faster than ever. Despite these leaps forward, the industry recognizes the pace is not sustainable due to the regulatory changes and cross-company collaboration it took to get there. To drive long-lasting change in trials, more work remains to modernize clinical systems, and contract research organizations (CROs) are leading the way.
CROs are taking significant action to speed study execution by investing in new digital strategies and technologies that bring together study processes in trials. In fact, 90% of CROs say they have initiatives in place to unify clinical operations, according to a recent study.{1} These efforts are driving more streamlined and connected ways of conducting research.
By increasing efficiency in major clinical areas and improving collaboration across the trial ecosystem, CROs are advancing the industry toward patient-centric digital trials. The ongoing modernization elevates the industry to a new level of connectivity that will benefit life sciences and patients for years to come.
Modernizing Across the Clinical Landscape for Faster Execution
CROs are implementing digital approaches across the clinical spectrum to speed trials. New solutions are making it easier to modernize specific areas, and there’s been an acceleration in the adoption of purpose-built applications.
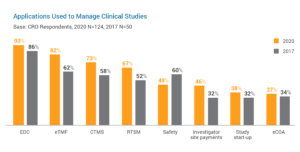
Study start-up is an area with significant potential to speed trial cycle times and improve overall efficiency in studies. This explains why 38% of CROs are using purpose-built study start-up applications, signaling a shift from manual methods like spreadsheets and e-mail to advanced solutions. The key drivers accelerating change among CROs are improving start-up times (73%) and reducing manual processes (52%). These advancements in study start-up will have a positive downstream impact on trials as more sponsors outsource early trial activities like site feasibility and selection to CROs.
In data management, one of the earliest areas to implement process automation, a growing number of leading CROs are looking to innovative clinical data management applications to run higher quality studies for sponsors.{2} This new breed of solution enables study builds in six weeks or less and delivers the agility to satisfy even the most complex data requirements.
CROs are also mitigating delays and issues in studies much quicker than ever before. With complete study oversight, clinical trial management systems (CTMS) empower CROs to identify problem areas and make instant decisions. This form of proactive trial management increases efficiency for CROs and research sites alike. The shift from spreadsheets and homegrown systems to digital clinical trial management has been key in standardizing study processes for faster execution.
Trial master file (TMF) management is undergoing vast transformation as more CROs move to eTMF applications with advanced digital and collaboration capabilities. Modern eTMF is driving positive improvements for CROs in TMF accuracy (70%) and completeness (63%) over other methods like paper and local file systems. eTMF is a vital tool for improved information sharing and better collaboration in trials.
Automating Information Exchange for Improved Collaboration
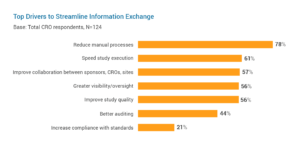
An overreliance on paper and manual processes in clinical trials make it difficult to share information with key stakeholders. The growing complexity and increasing volumes of data in studies are making it even harder. For CROs, streamlining information sharing is a top priority to reduce manual processes (78%), speed study execution (61%), and improve collaboration with sponsors and sites (57%).
CROs are breaking down the paper barriers by adopting solutions that automate information flow. These new technologies connect investigator site systems with CRO clinical operations to streamline processes like safety letter distribution and study closeout transfers. By transforming information exchange from manual and paper-based to digital and automated, CROs are improving collaboration in studies.
Investments in decentralized approaches are also improving information sharing across trial stakeholders. For example, informed consent has been traditionally managed manually on paper. Solutions like eConsent digitize the process; eConsent makes it easier for patients to understand and provide informed consent and for CROs and sites to share information. More CROs are enabling sites to use eConsent for a simpler, paperless consenting process.
CROs are Digitizing Processes and Connecting Systems for Faster Trials
Sponsors outsourcing clinical trials expect to gain access to innovative technologies and approaches. To offer differentiated services and deliver efficiencies to sponsors, more CROs are digitizing processes and connecting systems. During the pandemic, this proved crucial to ensure continuity in trials. Many companies experienced delays because study monitors could not verify data at research sites. The CROs that unified study documents and data, and had the digital capabilities to enable remote monitoring, kept trials moving forward.
Looking ahead, these same organizations embracing digital will speed trials and lower overall costs, delivering transformational change for the industry. We’re only at the tip of the iceberg, but the advancements will be swift. Thank you, CROs, for moving the industry toward future trials that are digital, connected, and fast.
References
- Veeva Systems. 2020. Veeva Unified Clinical Operations Survey: Annual CRO Report
- Veeva Systems. 2020. 6 of the Top 7 Global CROs Partner with Veeva to Run Faster Clinical Trials for Sponsors

Jim Reilly is Vice President for Vault R&D with Veeva Systems. For the last 15 years, he has held a variety of senior positions in life sciences technology, leading software delivery and sales in clinical operations, regulatory, clinical data standards, and content management. He is responsible for customer engagement, market adoption, and strategic alliances across the entire Veeva Vault R&D product portfolio.



