Clinical Researcher—June 2023 (Volume 37, Issue 3)
FORM & FUNCTION
Donna Conroy, MS
In the December 2022 issue of Clinical Researcher, I penned an article introducing the concept of Development Velocity (DV), the speed at which a pharmaceutical company moves a new drug through the development process. In the months since, new information has emerged on the widening financial chasm pharma is facing on two fronts: increasing development costs and diminishing market exclusivity.
Together, these challenges validate the need for pharma to improve DV or face difficult decisions about reducing research and development (R&D) expenditures; decisions with life-altering implications for patients.
Strategic end-to-end drug development, a lesser-known component of the R&D process that occurs in parallel with clinical trials, represents a major opportunity for pharma to recoup costs and find critical time efficiencies. By measuring and improving DV in this untapped area of R&D, pharma can begin closing the financial chasm.
Our research found that adopting technology to increase DV with data integration and workflow automation tools alone can save tier-one pharma companies an average of $202 million and 212,000 person hours annually. DV’s purpose is to get drugs to market faster. Earlier revenue streams are beneficial and can create competitive advantages that help drugs win “first to market” status and benefit from the additional and well-documented 6% market share advantage.
Pharma’s Expanding Financial Chasm
Deloitte recently reported the average cost to develop a new asset climbed to a staggering $2.2 billion in 2022, an increase of $298 million from 2021. Further, the top 10 drugs that lost market exclusivity in 2022 together generated $17 billion in yearly sales.
Refilling the pipeline is becoming increasingly challenging, as macroeconomic changes impacting deal flow are starting to have downstream implications—such as fewer acquisitions of innovative products. These factors create a feedback loop where existing drug development programs are under ever-increasing pressure to move faster to help offset lost revenue and rapidly rising costs.
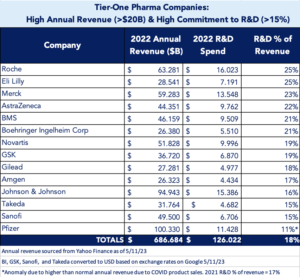
When we drill down, the financial benefits of accelerating development become clear. Tier-one pharma companies (see chart), defined as those with high annual revenue (>$20 billion) and high commitment to R&D (>15%), saw combined annual revenue of $687 billion in 2022, with an average 18% of revenue, or $126 billion, dedicated to R&D efforts. Our research found these companies collectively spent an estimated $2.8 billion just on data analytics to support strategic end-to-end development efforts (range $69 million to $359 million per company; average $201 million) in 2022.
Introducing comprehensive artificial intelligence (AI) technology into this R&D component can reduce data analytics costs by 67% or $1.9 billion (range $58 million to $251 million per company; average $135 million) by eliminating inefficiencies such as redundant analytics, slow and manual analytics, and siloed analytical technologies. Factor in additional savings from project management efficiencies like aligning data findings to specific tasks, sharing information, and linking interdependencies, and the cost savings increase significantly.
The benefits transcend both ends of pharma’s ledger, with earlier revenue generated from drugs that get to market faster and the potential to tap into the 6% first-to-market advantage. Together, these factors help pharma close the financial chasm.
Pharma’s Financial Chasm Impacts Patients, Too
The impact of this growing financial chasm may affect healthcare in general. With costs to develop new therapies increasing and market exclusivity decreasing, overall return on investment (ROI) is lower. Shrinking ROI may force pharma to reduce R&D budgets, resulting in less availability of new therapies or fewer studies in expanded patient populations. Our early analysis shows a slight drop in R&D investment in 2022 (currently 18%; down from 19% in 2021), however more data and time are needed to fully evaluate if this is due to shrinking ROI or factors related to the COVID-19 pandemic (as was the case for the Pfizer anomaly seen in the chart above). This is a trend we are tracking.
DV Measures an Untapped Value Opportunity Within R&D
Strategic end-to-end drug development refers to the cross-functional work required to move a drug to market—work that takes place after drug discovery and outside clinical trials. Despite investing billions in AI technology to enhance drug discovery and clinical trials, strategic end-to-end drug development remains untapped with no comprehensive innovation; it is laden with manual and siloed legacy processes. The lack of comprehensive data integration and workflow automation tools creates inefficiencies that slow the development journey and increase costs.
While most pharma companies apply some internal key performance indicators to measure efficiencies within strategic end-to-end development, the depth and breadth vary wildly. It is important for DV to be adopted as an industry-wide, third-party, mission-critical metric. The main benefit being that DV is a standardized process that normalizes data across drug programs, both within a company and industry wide. Situational components and variables, large and small, are accounted for among development programs. DV considers factors such as disease size, available datasets, team size, and funding, to name a few.
The DV Index score is similar to a FICO score: it assigns different weights to variables that impact performance. In the FICO example, the credit rating of a college student is normalized to provide a score that can be accurately compared to a long-time homeowner with established credit. By normalizing data in similar fashion, a rare disease and cardiovascular disease can be evaluated on an equal plane, despite rare diseases typically involving significantly less data and smaller teams. DV accounts for these anomalies in its scoring methodology to provide a uniform measure of time and cost efficiencies across drug development programs.
DV = Efficiency + Time
DV utilizes a proprietary formula that provides an impartial and unique assessment of a drug development program’s comprehensive operational efficiency. While current drug development efficiency models only consider the financial efficiencies, DV measures both financial efficiencies (i.e., cost savings) and time to market.
Time to market is significantly impacted by the hours it takes to accomplish more than 300 required tasks in a product’s development roadmap. Inefficiencies and redundancies result in hours wasted by pharma and its vendors. In 2022, our research found tier-one pharma companies collectively spent an estimated 3 million hours on data analytics alone (range 75,000 to 391,000 hours per company; average 212,000 hours) for various roadmap tasks in different development phases of their pipeline programs.
With comprehensive, cross-functional technology, pharma could have completed those same data analytics in 117,000 hours. In other words, in 2022 alone, pharma collectively wasted 2.9 million hours (range 72,000 to 329,000 hours per company; average reduction 203,000 hours). Assessing a drug program’s velocity is a critical component of DV. Reclaiming time helps accelerate therapies to market while closing the financial chasm.
DV measures more than 200 critical factors that foster inefficiencies. They fall into three primary categories:
Data Utility: How science is used for decision making
Example: Redundant literature searches among cross-functional teams
Time Inefficiency: The same base of data from 50 publications was used to answer questions across teams. Data findings were not shared across teams, resulting in redundant work hours.
Cost Inefficiency: Vendors typically charge between $50,000 and $250,000 to conduct literature searches. Multiple charges were made to identify and analyze the same datasets.
Project Management: Effectiveness in orchestrating cross-functional teams and tasks
Example: Lack of collaborative tools limits the sharing of interdependent deliverables
Time Inefficiency: Hunting for documents that inform workflow, whether on SharePoint or directly from a colleague, is time-consuming.
Cost Inefficiency: Multiple technology purchases to accommodate the needs of individual functions.
Corporate Approach: The role of corporate culture in strategic development
Example: Inability to pivot a strategy quickly when competitive data releases.
Time Inefficiency: Without keeping everyone up to date in real time, the process for quick cross functional decision making is laden with multiple steps.
Cost Inefficiency: Time to market is threatened and may impact overall revenue.
The impact of inefficiencies varies; some slow development by only a few hours or days, while others derail progress for weeks or months. However, in a multi-year drug development program, even seemingly minor inefficiencies compound over time to slow development timelines and increase budgets.
Inefficiencies, once identified, ranked, and scored, must be reduced or removed for meaningful change to occur. Our industry must overhaul the strategic end-to-end development process and utilize emerging technology and tools built specifically to address the needs of cross-functional drug development teams. Replacing antiquated processes with AI technology to improve data integration and workflow automation will accelerate strategic development and provide a resource to begin closing the financial chasm.
Conclusion: Why Focus on Efficiency in Drug Development?
Drug development is expensive and time-consuming, but it is possible to create efficiencies that offset the financial chasm of increasing costs and diminishing market exclusivity currently impacting pharma. By focusing on improving DV and incorporating AI technology into strategic end-to-end drug development, every pharma company can create game-changing efficiencies in R&D.
Committing to innovation will open the doors to urgently needed cost and time savings in an era when pharma faces unprecedented fiscal pressures. Patient lives are at stake and strategic drug development offers an untapped solution to drive innovation in R&D, one that allows teams to fail fast or succeed fast. The time to embrace innovation in drug development is now.

Donna Conroy, MS, (donna@scimarone.com) is Co-founder and CEO of SciMar ONE, a technology company that enables the pharmaceutical industry to accelerate drug development through an AI-based software as a solution platform.



