Clinical Researcher—December 2023 (Volume 37, Issue 6)
PEER REVIEWED
Elizabeth Anne Johnson, PhD, MS-CRM, RN; Lisa Marsh, MA, BSN, RN
Clinical trials are defined as the “testing of potential treatments in human volunteers to determine if drugs, medical devices, or biologics are safe and effective and should be approved for the general population.”{1} As we write this, more than 430,000 clinical trials are actively being conducted or have completed enrollment in the United States and 221 countries.{2} Each of these clinical trials is the rite of passage for a new treatment or therapy as it undergoes extensive safety evaluation, applicability for the intended population or disease of interest, and demonstration of benefit compared to standard-of-care, in terms of approved drugs already integrated into patient care pathways.
In 2022 alone, the U.S. Food and Drug Administration (FDA) reported that 38,000 clinical trial participants enrolled in U.S.-based research, contributing to 50 new, approved therapies to be available for commercial use.{3} Each of these novel therapies, made possible through the volunteerism of trial participants, is the culmination of an average of 10 years of research and development.{4}
While traditional clinical trial models are conducted at a research site, such as a hospital or clinic, the acute phase of the COVID-19 pandemic brought in-person, non-essential interactions to an abrupt halt. Many hospitals were only allowing emergency admissions and critically necessary patient care, with most of clinical research activity not related to COVID-19 research placed on hold. Nurses belonging to specialty of nursing dedicated to clinical trial conduct, known as clinical research nurses (CRNs), were either furloughed, transitioned to inpatient care assignments, or tasked with adapting current clinical trials to remote or virtual means of completing participant safety assessments and investigational drug administration.{5}
This transition to what is known as a decentralized clinical trial (DCT) model involved new software platforms, use of video conferencing services like Zoom, and electronic consent and new ways to communicate with participants not setting foot in the facility. Even with clinical trial participation returning to in-person encounters for most research sites, the benefits of decentralized trials emerged as means to address longstanding issues in research. Decentralized trials permitted participants to remain in their communities, access local providers for care, and have research visits completed in the comfort of their home without potentially long and costly travel to far away research site.{6}
The DCT model extended the opportunity to receive cutting-edge investigational products to populations otherwise unable to shoulder the financial and commitment burdens of clinical trials, advancing the initiatives of equity and representativeness of diverse peoples in drug and device data.{7} The model is here to stay: faster-paced recruitment of participants and delivery of a better trial participation experience has equated to previously delayed trials becoming now viable for enrollment and more studies including remote components in the trial design.{8}
This paper describes the practice changes that CRNs, who perform direct patient care, may anticipate when DCT participation is involved, considering the CRN as a supportive role for clinical nurse care delivery guidance, identifying trial participants, and obtaining safety-related study information to inform care and ethical considerations. We also examine current trends in DCTs which affect clinical nurses.
Clinical Research Nurses: A Research Lifeline and Liaison
A lack of clinical nurse awareness of clinical trial protocol–related restrictions on patient care could lead to medication errors, patient injury, or death.{9,10} For example, there are some experimental cancer therapies which interact with ondansetron, which is commonly administered for nausea. Instead, the clinical nurse may need to administer a different type of anti-nausea drug to deter widespread systemic drug interaction effects, which the research team could provide as part of information exchange while the patient is under a facility’s clinical care.
A recent literature review identified a need for clinical nurse education surrounding clinical trial conduct, an exploration of its tangent to patient advocacy in clinical settings, as well as establishment of effective communication with the study team and clinical leadership across the continuum of patient care.{11} To be clear, clinical nurses are not responsible for conduct of the clinical trial, however they benefit from an awareness of the patient’s trial details at time of obtaining the medical history to provide the best possible patient care. For example, there may be dietary restrictions for patients on certain investigative medications or additional focused patient assessments integrated into the care plan.
The CRN is typically the primary site contact for other healthcare providers or research personnel for the clinical trial and serves as nurse educator for the clinical trial to direct care nurses and multidisciplinary medical teams. CRNs who specialize in clinical trial–related work may be based in the hospital setting and conduct in-services or training meetings surrounding particularly common touchpoints such as nursing assessment, medication administration, and signs/symptoms to monitor. The CRN is an expert in trial conduct and participant management and is a skilled communicator to providers and nurses outside the research team for best approaches to ensure patient safety and retention in clinical in-patient units or in external settings, such as affiliated outpatient clinics.{12}
The CRN works closely with the principal investigator (PI) of the trial and apprises the PI as to any change in participant status. Operating the local clinical trial logistics makes the CRN a trusted community partner and integral research team member, connecting the PI to nearby providers for continuing conversations surrounding intricate care management.
Decentralized Participant Safety When Standard-of-Care Becomes Not-So-Standard
The flexibility in decentralized trial participation has generated high interest among cancer patients and patients associated with other diseases/disorders, with an estimated half of all patients being willing to enroll on a clinical trial.{7} For example, the number of oncology decentralized trials has risen 11% year-over-year, with almost half (46%) of all oncology trials including at least one component of remote or virtual-based participant management and/or data collection method.{8}
With more trial participants being able to remain in their home communities through the growth of decentralized trials, there is higher likelihood of clinical nurses to encounter patients who double as trial participants. However, clinical trial participants are easily camouflaged in the bustle of clinical tasks; wallet cards and other means of identification affiliated with the clinical trial are inconsistently implemented.{13} There is lack of reliable recall among participants to convey complex details surrounding what medications are safe for nurses to administer or which side effects are anticipated.{13,14} Further, electronic health record (EHR) systems are not standardized in their adoption for research-related use and may not include full integration with clinically facing modules.{14}
Safety-related details are necessary for the clinical nurse to navigate decision-making related to patient plan of care. Figure 1, derived from Hannawa’s essential communication framework and the Johnson, et al. clinical trial–specific communication framework, details steps to identify trial participants, obtain critical information, and interpret for clinical care actions.{15,16} Prompting each patient encounter with an inquiry such as, “are you participating in a clinical trial or research study?” initially identifies those patients requiring unique care considerations (ASK in Figure 1). Since a decentralized trial may mean the research team is not local to the facility caring for the trial participant, collecting research team details and initiating contact with the CRN will support education and guidance surrounding safe patient care (CONNECT in Figure 1). For example, many investigational drugs and devices may not have assigned names, being identified instead by alphanumeric identifiers that cannot be traced in common drug databases.
New technologies may be discovered, such as new remote patient monitoring devices or wearables.{6,17} What is considered standard-of-care may be incongruent to the clinical trial protocol. If not in adherence to the trial expectations, the participant may be withdrawn, resulting in loss of opportunity and access to potentially lifesaving products. There may be a period of “interactive sense making,” or trying to understand how the clinical trial is significant for patient care and what details are important based on input from the patient and the CRN.{15,18} Documentation of clinical decision-making steps and fidelity of the care plan related to the trial protocol will support research team management of the participant. This includes dose adjustments or additional follow-up visits to monitor an adverse event (CARE in Figure 1).
Figure 1: Recommended Steps for Clinical Management of a Patient Participating in a Decentralized Trial
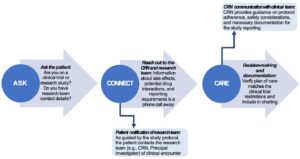
CRNs may support clinical nurses through other modalities of obtaining clinical trial information important to care delivery, such as tipsheets or binders of key details left at nurse stations. Some facilities may use special icons, different colored chart flags, or wristbands to help clinical nurses and other healthcare providers distinguish clinical trial participants from “regular” patients at the facility.{17}
Clinical nurses are recommended to be watchful for dashboard notifications in the EHR and corresponding research-related modules that may require review so that medications, assessments, and frequency of patient status checks are aligned to the type of clinical trial and alterations to normal laboratory values—for example, due to an investigational drug or device.
Implementation Considerations
Decentralized trials expand trial access to settings outside traditional hospital or research units, which comes with a unique set of ethical challenges related to institutional review board (IRB) review and PI oversight. During the COVID-19 pandemic, decentralized trial models or a mix of traditional (at a facility) and some decentralized (such as blood draws at home) activities were encouraged to continue participant access to novel treatments while adhering to federal guidelines for minimizing interpersonal contact.{19}
In 2021, the FDA issued guidance on how to navigate regulatory hurdles without the structure of organizational bounds of a hospital. Per the guidance, a research team is delegated to ensure safety of participants in the decentralized model in collaboration with their respective IRB to support contextual adaptations to how to control for varied resources in home-based or community research conduct.{20}
Decentralized trials require close partnership with participants, caregivers, and clinical providers to minimize risk or potential for harm.{21} Partnership includes PI oversight of local provider engagement strategies and communication channels, which includes the CRN as the backbone and liaison for the community and participant. Effective communication between clinical nurses and CRNs supports participant safety and quality in recorded outcome data, which supports new drug and device applications to be approved for commercial use.
Informed Consent, Ethical Challenges, and Communicating Safety Information
While the PI, CRN, and research team are ultimately responsible for supporting communication of clinical trial participant safety information to clinical teams, the clinical nurse holds a critical role in advocating for safe care aligned, when possible, to the research protocol. Per the American Nurses Association Scope and Standards of Practice published in 2021, Ethics Provisions 2 and 3, the nurse’s primary commitment is to the patient which includes promotion for health and safety. Standard 10 notes that the nurse must also be able to effectively communicate across all areas of practice which includes ancillary medical teams as well as the patient.{22}
Communication is the cornerstone to quality patient care, and poor or ineffective communication between research and clinical teams may result in harm to participants and stress for the clinical nurse.{23} An ethical challenge pertaining to communication of safety information is the informed consent process and how best to support participant understanding of protocol expectations, when to communicate their involvement in a clinical trial to the clinical nurse, and how informed consent affects clinical nursing practice. Particularly when not accustomed to integrating research-related safety considerations in the clinical setting, nurses have reported distress when trying to manage an adverse event and the extra work of unique care requirements with a participant.{24}
Meanwhile, participants historically have been generalized as having significant challenges understanding trial material presented within informed consent forms, which can be at a 10th-grade reading level and confusing due to detailed information presented in scientific terminology.{10} Tam, et al. reported across 103 studies and 135 cohorts of trial participants that only 54.9% could name at least one risk associated with participation on the clinical trial.{25} While wallet cards are commonly associated with clinical trial communication of safety and contact information to clinical teams, Schoenenberger-Arnaiz, et al. found that, across 67 protocols, 37% did not mention the card or its use in the event of clinical care within the informed consent form.{13}
When confronted with ethical challenges such as those with balancing integration of trial safety information within clinical care workflow, clinical nurses have reported that support from colleagues, such as CRNs, aids in the navigation of clinical decision-making. Clinical nurses have also reported a sense of pressure and feeling inadequately informed or trained to manage clinical trial participants as patients, which is compounded with communication gaps and participant knowledge deficits. Routine engagement with CRNs and details pertaining to the informed consent form and the process of consent were additional supportive structures to aid clinical nurses in feeling more prepared during encounters with trial participants.{24}
The CRN, PI, and other members of the research team are delegated by a trial sponsor to support ethical and optimal trial conduct. This delegation and responsibility then translate to ensuring that clinical nurses feel supported in managing participant care outside the study. Research team actions can include simplification of informed consent form language for participant understanding as well as education in-services for nursing staff of hospital systems pertaining to trial participant clinical management strategies.{10,25}
On the Horizon: Future Trends in Decentralized, Remote Research
The influx of localized participation in clinical trials and research studies has sparked integration of research-related services among companies with pre-established presence in metropolitan and non-metropolitan settings. Following the acute phase of the COVID-19 pandemic, healthcare titans CVS Health and Walgreens announced a strategic focus on clinical trial participant recruitment via their pharmacy and clinic locations, leveraging the existing brick-and-mortar community footprint and customer traffic to create awareness of decentralized trial opportunities. Both companies communicated their commitment to address a common issue of DCTs surrounding investigational product management, which is the shipping, storage, and dispersal of experimental drugs in the event the local research site does not have the necessary resources.{26,27}
As almost 4 out of 5 Americans live near a Walgreens, there is high potential for more diverse, representative enrollment of trial participants, lending enhanced real-world data to inform new drug and device application reviews prior to FDA approval.{27,28} While CVS Health will depart the decentralized trial space by 2024, Walgreens and other healthcare or pharmacy-based companies have retained their resolve to support remote participant trial recruitment through expanding their programs into primary care settings, urgent care, and retail locations.{29}
The quest for diverse patient population representativeness in clinical trial data includes geographic diversity, which includes the enrollment of suburban or rural-dwelling residents to better understand the pragmatic, more logistical aspects of a novel drug or device’s use in areas which are lacking in terms of healthcare resources.
In October 2022, Walmart announced that it too was entering the clinical research space, with a focus on increasing access to clinical trials and heavy adoption of mobile, virtual technologies (such as a mobile app) to pair pharmacy and clinic patients to trial opportunities corresponding to their health information.{30} Retailer Dollar General also is leveraging its heavy presence in rural areas, launching thousands of pilot mobile clinics in some stores as part of its healthcare debut.{31} The staffed mobile clinics, called DocGo On-Demand, signal an overarching change in community-focused healthcare delivery, broadening of consumer healthcare options, and potential access points of decentralized trial patients outside hospital-based facilities.
As these retail and commercial locations are found across both urban and non-urban centers, they provide potential job opportunities for CRNs given their specialization and expertise. The inclusion of non-traditional study sites also may be integrated as resources to facilitate trial procedures (e.g., specimen collection, physical assessments, or investigational product dispensing) closer to the participant’s location, which may create workflow efficiencies for CRNs in visit scheduling and staff allocation.
Nurses working in suburban and rural community clinics, or in freestanding emergency/urgent care facilities, may be contributing to the conduct of clinical research in cases when DCT participants require care or completion of procedures outside the research setting. For example, a participant may experience a side effect or critical symptom requiring medical attention. Having a clinical nurse attuned to assessing for trial participation in a non-hospital setting benefits the patient to ensure safe care congruent to the protocol restrictions. The clinical nurse may then also contact the CRN of the research study for more information related to adaptations to nursing assessments and explanations of signs or symptoms attributable to the study drug or commonly seen on the trial.
Particularly in lower resourced settings, such as critical access hospitals, clinical nurses will benefit from quick touchpoints with a CRN on how to adapt complex investigative product management within the constraints of the extend of care traditionally provided. For example, the CRN may work with the clinical nurse on an interfacility transfer with documentation to a hospital with critical care capacity should a participant require a higher degree of supportive care.
Conclusion
In a recent survey of clinical research executives, almost 4 out of 5 noted use of a decentralized trial model within the next year as part of the development pathway of a new drug or device toward commercialization.{8} DCTs are here to stay, with potential to increase clinical trial access, diversity, and likely faster approval of new treatments.
As clinical trial participation becomes more mobile and accessible outside traditional research centers, nursing awareness of the unique care considerations of patient-participants becomes crucial. While clinical trial details are not readily available at point of care for many nurses, adjusting patient interviewing and adopting a situational awareness to the potential of encountering a trial participant (see Figure 1) can keep a community member taking an investigational product safe. CRNs are at-the-ready as skilled communicators of critical safety and reporting information in such situations, balancing clinical experience with trial expertise to assist clinical nurses in care decision-making.
For many patients, each clinical trial enrollment is a hard-fought opportunity to keep hope alive when other treatments are not effective. Widening trial access and closing the barrier gap among under-represented populations prompts agility across the nursing profession to extend patient advocacy to those enrolled in clinical trials. Creating a flexibility in the definition of standard-of-care beyond established treatments incorporates experimental treatments deemed appropriate for a patient’s particular disease process, disorder, or symptom. Made possible by decentralized trials, cutting-edge medicinal advancement has never been closer to home.
References
- U.S. Food and Drug Administration (FDA). 2021. Guidance for Industry, Investigators, and Institutional Review Boards: Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency. https://www.fda.gov/media/136238/download
- U.S. National Library of Medicine. 2023. Trends, Charts, and Maps. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/resources/trends
- FDA. 2022. Center for Drug Evaluation and Research. Drug Trials Snapshots. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots
- The IQVIA Institute. 2022. Global Trends in R&D 2022. Industry Report. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-trends-in-r-and-d-2022
- Van Dorn A. 2020. Covid-19 and Readjusting Clinical Trials. The Lancet 396(10250):523–4. https://doi.org/10.1016/s0140-6736(20)31787-6
- Van Norman GA. 2021. Decentralized Clinical Trials. JACC: Basic to Translational Science 6(4):384–87. https://doi.org/10.1016/j.jacbts.2021.01.011
- Unger JM, Hershman DL, Till C, Minasian LM, Osarogiagbon RU, Fleury ME, Vaidya R. 2020. “When Offered to Participate”: A Systematic Review and Meta-Analysis of Patient Agreement to Participate in Cancer Clinical Trials. JNCI: Journal of the National Cancer Institute 113(3):244–57. https://doi.org/10.1093/jnci/djaa155
- LaHucik K. 2021. About 77% of Clinical Research Execs Expect to Run Decentralized Trials in Next 12 Months: Survey. Fierce Biotech. https://www.fiercebiotech.com/cro/nearly-80-clinical-research-execs-expect-to-run-decentralized-trials-next-12-months-survey
- Ulrich CM, Knafl K, Foxwell AM, Zhou Q, Paidipati C, Tiller D, Ratcliffe SJ, et al. 2021. Experiences of Patients after Withdrawal from Cancer Clinical Trials. JAMA Network Open 4(8). https://doi.org/10.1001/jamanetworkopen.2021.20052
- Fogel DB. 2018. Factors Associated with Clinical Trials That Fail and Opportunities for Improving the Likelihood of Success: A Review. Contemporary Clinical Trials Communications 11:156–64. https://doi.org/10.1016/j.conctc.2018.08.001
- Hale A, Barton B, Serino‐Cipoletta J, Sheldon Y, Vessey JA. 2022. Direct Care Nurses’ Perceptions of Their Roles in Clinical Research: An Integrated Review of the Literature. Journal of Nursing Scholarship 54(1):104–16. https://doi.org/10.1111/jnu.12704
- Fisher CA, Griffith CA, Lee H, Smith HA, Jones CT, Grinke KA, Keller R, Cusack G, Browning S, Hill G. 2022. Extending the Description of the Clinical Research Nursing Workforce. Journal of Research in Nursing 27(1–2):102–13. https://doi.org/10.1177/17449871211068631
- Schoenenberger-Arnaiz JA, Solanilla-Puertolas M, Acer-Puig M, Gomez-Arbones J. 2017. Informing Primary Care Physicians of Patients’ Involvement in Clinical Trials Carried Out at a Specialist Care Level. Open Access Journal of Clinical Trials 9:59–64. https://doi.org/10.2147/oajct.s134555
- Johnson EA, Carrington JM. 2020. Clinical Research Integration Within the Electronic Health Record. CIN: Computers, Informatics, Nursing 39(3):129–35. https://doi.org/10.1097/cin.0000000000000659
- Hannawa AF. 2018. “Saccia Safe Communication”: Five Core Competencies for Safe and High-Quality Care. Journal of Patient Safety and Risk Management 23(3):99–107. https://doi.org/10.1177/2516043518774445
- Johnson EA, Rainbow JG, Reed PG, Gephart SM, Carrington JM. 2022. Developing a Preclinical Nurse-Nurse Communication Framework for Clinical Trial Patient-Related Safety Information. CIN: Computers, Informatics, Nursing 41(7):514–21. https://doi.org/10.1097/cin.0000000000000968
- Johnson EA, Rainbow JG, Carrington JM. 2023. Clinical Nurses’ Identification of a Wearable Universal Serial Bus Used for Pediatric Oncology Clinical Trial Participant Safety Management. CIN: Computers, Informatics, Nursing (published ahead of print). https://doi.org/10.1097/cin.0000000000001013
- Johnson EA, Carrington JM, Rainbow J. 2020. Nursing’s Role in Translating Safe Communication Practices to Clinical Trial Management. Proceedings of the Human Factors and Ergonomics Society Annual Meeting 64(1):1140–4. https://doi.org/10.1177/1071181320641273
- Petrini C, Mannelli C, Riva L, Gainotti S, Gussoni G. 2022 Decentralized Clinical Trials (DCTS): A Few Ethical Considerations. Frontiers in Public Health. https://doi.org/10.3389/fpubh.2022.1081150
- FDA. 2020. Conducting Clinical Trials. Development and Approval Process: Drugs. https://www.fda.gov/drugs/development-approval-process-drugs/conducting-clinical-trials
- Apostolaros M, Babaian D, Corneli A, Forrest A, Hamre G, Hewett J, Podolsky L, Popat V, Randall P. 2019. Legal, Regulatory, and Practical Issues to Consider When Adopting Decentralized Clinical Trials: Recommendations from the Clinical Trials Transformation Initiative. Therapeutic Innovation & Regulatory Science 54(4):779–87. https://doi.org/10.1007/s43441-019-00006-4
- American Nurses Association. 2021. Nursing: Scope and Standards of Practice (4th ed.). https://www.nursingworld.org/nurses-books/nursing-scope-and-standards-of-practice-4th-edit/
- Gorkun A. 2023. Improving Communication in Clinical Research. SOCRA Blog. Society of Clinical Research Associates. https://www.socra.org/blog/improving-communication-in-clinical-research/
- Godskesen TE, Petri S, Eriksson S, Halkoaho A, Mangset M, Pirinen M, Nielsen ZE. 2018. When Nursing Care and Clinical Trials Coincide: A Qualitative Study of the Views of Nordic Oncology and Hematology Nurses on Ethical Work Challenges. Journal of Empirical Research on Human Research Ethics 13(5):475–85. https://doi.org/10.1177/1556264618783555
- Tam NT, Huy NT, Thoa LT, Long NP, Trang NT, Hirayama K, Karbwang J. 2015. Participants’ Understanding of Informed Consent in Clinical Trials over Three Decades: Systematic Review and Meta-Analysis. Bulletin of the World Health Organization 93(3):390. https://doi.org/10.2471/blt.14.141
- CVS Health. 2021. CVS Health Introduces Clinical Trial Services. Other Health Care Services. https://www.cvshealth.com/news/clinical-trial-services/cvs-health-introduces-clinical-trial-services.html
- Japsen B. 2022. Walgreens Launches Clinical Trial Business as FDA Seeks Diversity of Patients. Forbes. https://www.forbes.com/sites/brucejapsen/2022/06/16/walgreens-launches-clinical-trial-business-as-fda-seeks-diversity-of-patients/?sh=2ea63930554e
- Walgreens Health. 2022. Re-envisioning Clinical Trials. https://www.walgreenshealth.com/clinical-trials
- Gagosz A. 2023. Walgreens Doubles Down in Clinical Trials Space after CVS Bows Out. Boston Globe. https://www.bostonglobe.com/2023/06/16/metro/walgreens-doubles-down-clinical-trials-space-after-cvs-bows-out/
- McLymore A. 2022. Walmart to Compete with Walgreens, CVS in Recruiting Clinical Trial Subjects. Reuters via Yahoo! News. https://news.yahoo.com/walmart-compete-walgreens-cvs-recruiting-104940216.html
- Landi H. 2023. Dollar General Pilots Mobile Clinics as it Targets a Bigger Presence in Healthcare. Fierce Healthcare. https://www.fiercehealthcare.com/providers/dollar-general-pilots-mobile-clinics-it-targets-bigger-presence-healthcare

Elizabeth Anne Johnson, PhD, MS-CRM, RN, (elizabeth.johnson37@montana.edu) is an Assistant Professor in the College of Nursing and Co-Director of the Biomedical Innovation for Research and Development (BioReD) Hub at Montana State University.

Lisa Marsh, MA, BSN, RN, (lisa.marsh@northwestu.edu) is a member of the Adjunct Faculty for the Northwest University Mark and Huldah Buntain College of Nursing, a Committee Member of the International Association of Clinical Research Nurses, and a Clinical Trial Lead with Boehringer Ingelheim.



