Clinical Researcher—May 2019 (Volume 33, Issue 5)
PEER REVIEWED
Jeff Lee
Imagine seeing a product you want to buy, but when you go to the website, it asks you to print an order form and mail or fax it in. Would you trust that provider or system with your credit card information? Probably not. And yet, that’s what we are asking patients to do with something far more important—their health—when it comes to clinical trials. People are used to technology, apps, and accessibility in their daily lives, so it is no surprise that a 2016 survey showed 31% of 137 responding patients reported themselves as more likely to participate in a clinical trial if it has a mobile app.{1}
In fact, Deloitte reports there are more than 260,000 health apps worldwide and the members of 70% of patient groups are using at least one app to manage their condition.{2} By utilizing digital solutions, pharma companies can tap into these progressively health-aware consumers. However, those working in the industry, specifically within clinical trials, often feel that the patients in their studies receive a mixed experience of technology, and many simply feel overwhelmed by too many systems.
So why isn’t the pharmaceutical industry moving more quickly to adopt solutions that patients—and sites and study teams—want? Barriers to digitally focused patient centricity include uncertainty over regulators’ expectations and requirements, and data safety and privacy concerns with the rise of medical apps and other digital technologies. Such trust issues can result in patients being unwilling to engage with pharma, while low levels of health and digital literacy also affect the ability to engage with patients effectively. Attracting the right talent to support a patient-centric ecosystem can also be a stumbling block, with a traditional product-based culture causing barriers to an agile, patient-centric approach.
Increased willingness to take a patient-centric approach to technology adoption to improve data capture, patient understanding, and patient engagement is needed to drive real return on investment for retention, compliance, protocol adherence, and overall study timelines. The first important step is improving adoption, but equally important is seamless integration and a single, unified user experience for the patient and sites. It is not enough to give them technology—it must blend into their daily lives so that it doesn’t get in their way. In other words, we seek easy-to-use solutions built with patients (and how they will actually use the tech) in mind. This article will therefore outline how electronic clinical outcome assessment (eCOA), electronic informed consent (eConsent), and patient engagement apps can work together in one solution to combine to transform the patient experience in clinical trials.
As an aside for readers who are concerned that their patients may lack the technologies necessary for the purposes described in this article, eCOA and eConsent are commonly offered using provisioned devices, so that all patients can utilize them, without regard to their personal mobile phone usage. Patient engagement is typically offered to patients on their personal devices, with either mobile app (for smartphone users) or SMS (text messages) for non-smartphone users. SMS works on any mobile phone, so this ensures that patient engagement can be provided to virtually any patient.
Creating a Seamless Patient Experience
In clinical studies, it is important to employ approaches that enable the optimal assessment of the study concepts of interest. Where this involves use of a technology solution, the aim is to simplify processes, make participation easier, improve quality, facilitate decision making, and collect reliable, honest data.
Patients and their families are understandably impacted by technology as they are going through a trial. In a typical study, for example, one patient may experience four devices, such as a wearable, a reminder app, eConsent, and an e-Diary. It is not uncommon for a trial participant to also use a payment and reimbursement website, all accessed via different systems and devices. As new technologies emerge, they appear via specialist vendors, so for each software and device, patients have a learning curve, different access points, and compliance requirements. As a result, the patient is faced with a dizzying array of disparate communications, devices, and systems, and if the process is too complex, the patient is more likely to quit the study and deviate off protocol. Seamless integration and a single, unified user experience are again the key to making participation as easy as possible without overburdening the patient. It might seem obvious, but they only need the tools that they need.
Sites today are leaning on technology help desks more than ever in their struggles to provide patients all the training they require. Fortunately, by combining these technologies into a single project delivery team, sites receive streamlined and coherent training, which makes them more effective educators to patients.
However, it is not just the patient who needs to be considered, as technology has an impact from multiple perspectives. Improving the clinical trial experience for all involved is a priority. For study teams, enabling them to see real-time study progress and improve efficiencies is the top priority for managing the complexities of a trial and not adding to it. Transparent, real-time insight is the key here. Meanwhile for management, any solution should improve oversight, efficiencies, and successful outcomes for return on investment.
Less Vendor Focused, More Solution Focused
As mentioned, seamless patient experience requires successful collaboration across many different areas of a trial—outreach, recruitment websites, screening, verification, eConsent, registration, data collection, data return, and incentives. How to bring all these elements together needs to be considered carefully, especially in a virtual trial where there may be no physical site. Figure 1 demonstrates what successful collaboration could look like.
Figure 1: Coordination Among Multiple Systems Sets a Higher Bar for a Seamless Patient Experience
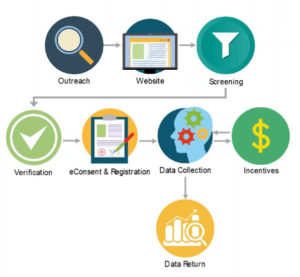
eConsent, eCOA, and patient engagement solutions are the cornerstones of a dependable, patient-centric technology solution to help improve the speed, reliability, and insight of clinical research. Combined into an integrated solution, it makes for a powerful approach.
eConsent
Patients need to be informed and retention starts on the right foot through the informed consent process. eConsent sets the patient up to fully understand the study well, and the engagement aspect carries them forward. eConsent incorporates patient-friendly features such as familiar and convenient mobile devices, multimedia video education, pop-up glossary terms, digestible electronic consent form sections, clearer assessments, and enhanced accessibility (i.e., audio narration, large fonts, etc.). There is a growing body of evidence to support the idea that eConsent improves patient comprehension and study retention. One study{3} demonstrated an increase in assessment understanding with eConsent education; meanwhile, CenterWatch{4} highlighted that it is possible to enroll 25% fewer patients to reach the same completion goals as paper-based studies. In addition, consent-related deviations are the second most common audit findings (e.g., people consenting on the wrong version, not re-consenting to new protocol amendments, etc.). eConsent practically eliminates these deviations and improves compliance, thus delivering major benefits in time and cost at the end of a study.
The benefits also extend to study teams, offering advantages such as real-time consent monitoring, bring-your-own-device (BYOD) implementation, SaaS content system creation (with customers configuring their own programs rather than relying on the platform provider for implementation services), eSignature, print-to-sign functionality, and a fully validated solution that provides value anywhere in the world.
eCOA/ePRO
With patient engagement–focused eCOA and electronic patient-reported outcome (ePRO, in which patient surveys are collected to help assess responses to the therapy that can’t be measured by lab tests) technologies, the patient has a better experience within the study, which underpins the ultimate goal of capturing the most reliable data and achieving higher retention rates and improved protocol compliance. Whilst a paper-based option could seem easier for patients to use to record information whenever needed, eCOA forces them to respond correctly, with guided responses, and only during the times specified by the protocol, thus preventing “junk data” from being entered at whim or convenience and misleading sponsors.
Access to reminders about personalized medications, appointments, and other study-specific details, benefiting from a single, mobile touchpoint that integrates study commitments into their daily life, are extras that increase engagement among patients. Content libraries presented in digital form, including video, PDF, audio, images, and text, can also facilitate comprehension and help keep the patient fully informed about the study. Bringing these together is an important way to guide patients through their study experiences—giving them the information they need all in one place. This is crucial in a virtual study, which offers less “hand holding” to patients from the site, making the reliance on technology more important.
Patient Engagement
Engaging a patient on a mobile device may drive better patient outcomes, compliance, change behaviors, etc. In the arena of mobile health, it is obvious that technology is vital, but it is important to look at the tools in terms of the value they deliver. Effective patient engagement solutions should eliminate the need for patients to access multiple disparate systems or rely on manual paper practices to keep up with study requirements. For study teams, mobile programs should be configured to match specific study protocols, work across all smartphones and tablets, and be deployable in a BYOD model.
Such a single, streamlined experience has numerous benefits for all stakeholders, and is a significant aid to making evidence-based decisions. Successful patient engagement apps preferred by patients are ideally integrated into a seamless solution providing a much-simplified experience. They integrate visit reminders, documents, site contact info, critical updates, reimbursements, and courier services (e.g., Uber) into a single app. So even when multiple third-party providers are delivering the services, it is invisible to patients because it is all behind the scenes of the user-friendly app. The aim of technology should be to provide a better patient experience. Increasingly complex protocols are challenging enough without overwhelming technologies, plus a single patient experience inspires confidence in overall study conduct.
A streamlined experience is also simpler for sites, requiring less effort but greater impact. Single sign-on and data sharing across systems fosters simplicity and efficiency for site staff, while centralized data entry reduces the possibility of erroneous data and can reduce operational time and cost. Patient engagement apps also offer advantages for sponsors, as trials run smoothly, with faster recruitment and improved retention. Apps can increase patient comprehension of the study and reduce patient burden, resulting in higher completion rates. Efficiencies for sites translate to more active sites, and faster enrollment and less manual data entry provides better data accuracy and integrity.
In a recent vaccine study, patients were offered the option to receive patient engagement messages on their mobile device. Those who chose to receive SMS reminders, including visit reminders and engagement messages, had a 50% lower drop-out rate than those who did not. This also correlated with higher completion rates in the study, demonstrating a significant opportunity to guide the patient. The added benefit of this technology is that is can be used on both a patient’s own device and a provisioned device (BYOD). In a virtual study model, a personal device is more common, making it easy for the patient to download an app.
A First for Patients—Combining ePRO, eConsent, and Engagement
Patient engagement is fundamental not only within a traditional clinical trial setting, but also within a virtual trial, offering a new method of collecting safety and efficacy data from clinical trial participants. Virtual trials (also referred to as direct-to-patient, siteless, or remote trials) are decentralized trials that are less site-centric. The benefits of such trials are that they increase access to include hard-to-reach patient populations (i.e., new geographies) and allow the patient to perform study requirements independently away from the site. Challenges include drug supply, identity verification, consent, and more. Virtual studies are poised to take full advantage of the technologies that are out there, and this is where the value of collaboration and integration really comes into its own.
Case Study—An Integrated Solution
A recently created study sponsored by a top 10 pharmaceutical company looked at developing a deeper understanding and body of evidence around quality of life (QOL) metrics for a specific indication. There is a lot known about patient experience measured by biomarkers; however, the sponsor believed that the therapy has benefits to the patient that extend beyond optimizing the biomarker measure response. The sponsor wanted to conduct a health-related, QOL virtualized, observational study to attract a large number of patients with this indication and engage with them, with the goal of learning more about their QOL with this indication. Recruitment began in January 2018 with the goal of recruiting up to 1,500 patients. The sponsor was looking for an integrated technology solution to improve patient retention and identity verification. They wanted a solution that increased verification accuracy and reduced patient effort while increasing their comprehension of the study.
The integrated, end-to-end solution for patients was to be a second-generation initiative which presented opportunities and objectives to address challenges experienced in the previous trial, including:
The Challenges
- Patients were quick to join the program, but many did not persist (very high drop-off rates)
- A lack of patient engagement and support through the program
- Sponsor needed a reasonable assurance of each patient’s identity/medical condition
Key Requirements
- Take consent to the next level by increasing patients’ comprehension of the study
- Do more than collect data
- Improve patient retention
- Be patient friendly
- Follow a similar model to the app store that patients are familiar with to download an app
- Take specific care verifying the patient’s medical condition (via disease-specific screening questionnaire)
- Keep the patient’s interest through to completion
The Solution
The gold standard approach encompasses digital outreach, patient discovery via a landing page and a study overview section, screening, and eConsent through to an engagement app via the patient’s own device, making the process as easy as possible for the patient.
Such a solution offers a much-improved experience to patients and study teams. For patients, it offers a seamless experience, with less work and fewer systems and interfaces, whilst also building trust and increased understanding of the study. Patients are seamlessly guided through the process of learning about a study, understanding their eligibility, consenting, and beginning their engagement through their mobile device. The BYOD diary/patient engagement tool was quickly set up via an automatically initiated activation message sent directly to the patient’s phone. An effortless patient activation process takes the burden off sites and patients. Ease of use and rapid patient setup process instills confidence in users, as the system is intuitive even to first-time users. For study teams, this results in faster recruitment and better retention, higher conversion rates, and better diary completion rates. Full launch documentation was provided, including ethics committee/institutional review board (EC/IRB) submissions, data protection statements, and testing documentation, making deployment virtually effortless for the client team.
Despite targeting a niche patient population, the study reached its enrollment goal well before the target “last person in” date and well under the original outreach budget. Completion rates of the consent form suggested a good balance of user experience and rigor of consent form.
Automated study reminders kept patients on track with the protocol and reduced the burden on clinical research sites who would ordinarily remind study subjects to fulfill their commitments to the program. The solution delivered protocol-specific reminders about study medications, appointments, and PRO diary windows, as well as other need-to-know details. These were sent via text message, push notification, e-mail, or voice.
Completion rates (for both the diaries and the overall study experience) were approximately 10 times higher than the predecessor study, significantly exceeding the expectations of the sponsor.
Some Thoughts About Privacy
Our industry is faced with a complex and ever-changing privacy and data security landscape. Each provider of patient-facing technologies approaches these requirements differently, which creates a challenge for all parties involved. Sponsors expend significant effort auditing/qualifying each provider. Sites (and their EC/IRB groups) need to assess and verify that patient privacy rights are upheld by each provider. Patients need to reach a level of comfort with the idea that the multiple technologies with which they are interacting are all safe and secure.
By bringing all of these technologies together, this landscape is greatly simplified for all parties. Additionally, given the scale of providing multiple solutions, a larger organization can more easily stay fully up to speed on changing regulatory frameworks and legal requirements across the many geographic territories in which research is conducted.
A Future-Proof Gold Standard Solution
There is huge potential for thinking differently about how existing technologies can be utilized to enable novel measurements for health outcomes and health status in patients, while making the process as easy as possible for patients to participate in a trial. The goal is to move the industry beyond paper, for its obvious limitations and risks, while navigating the current landscape of decentralized, fragmented systems that do not talk to each other. First and foremost, we need to increase adoption, but just as important is the need to utilize technology in a seamlessly integrated way that fits into patient lives to collect life-changing insights without the trial hindering the process or the patient.
The vision of an integrated system with eConsent, eDiaries, reminder apps, and ePRO in one mobile solution that offers a unified solution—for improved patient experience with increased virtual capabilities—is here, but that is not the end. Novel use of technology encompassing wearable devices, smartphone sensors, and motion-based gaming platforms to collect new endpoints is coming along quickly. A future-proof gold standard solution is available through best-of-breed collaboration and pioneering solution integration intrinsic to this vision of transforming trials and the patient experience.
References
- PMG Research Web-Based Patient Panel Survey. August 2016.
- Pharma and the connected patient. https://www2.deloitte.com/bh/en/pages/life-sciences-and-healthcare/articles/pharma-and-connected-patient.html
- Rowbotham MC, et al. 2013. PLoS ONE 8(3).
- Moekel and Brady. 2003 CenterWatch Study referenced in https://www.drugdev.com/assets/Why-Isnt-Your-CRO-Using-eConsent.pdf.

Jeff Lee is Product Lead for Patient Engagement and eConsent with CRF Bracket.



