Clinical Researcher—June 2020 (Volume 34, Issue 6)
SPECIAL FEATURE
Ingrid Oakley-Girvan, PhD, MPH
The COVID-19 pandemic continues to have an unprecedented impact on individual lives, families, communities, healthcare systems, and economies around the world. For the public health and scientific community, there is an immediate need to accelerate COVID-19 research for diagnostic testing, immunity monitoring, and drug and vaccine development. At the same time, there is a critical need to ensure progress on existing clinical research for keeping active trials in flight while ensuring the safety of trial participants and healthcare workers.
As the COVID-19 pandemic has reinforced, traditional clinical trial processes and timelines are insufficient to meet the world’s need for safe, timely, and effective treatments. We believe the industry can and must move into a more decentralized approach to trials, including remote data collection via digital and mobile technologies, process innovations, and collaborative research approaches that span multiple stakeholders within the trial and research ecosystems.
This is possible because the technology is here, and has been for quite some time. However, until recently, virtual or decentralized trials were considered a progressive, “nice to have” option when designing a trial or research study. Traditional processes and mindsets dominated, despite the well-known issues of significant trial costs, slow timelines, poor enrollment, retention challenges, lack of participant diversity, and other challenges.
The tide is turning, though, and COVID-19 is a catalyst. Remote visits and mobile devices are absolutely vital now as people are expected to stay at home and keep their distance. Of the 55,000 trials in flight, some are good candidates for a fully decentralized model—while many others can be managed in a hybrid model. Patients can be recruited and consented remotely. Physician “visits” can often be conducted remotely via telemedicine. Data can easily be captured remotely (and frequently) via medical devices and mobile technology.
The ACCESS Initiative
While decentralized trials provide both a short- and long-term solution to ensuring clinical research progress, we also believe the industry needs to adopt more creative and collaborative approaches to research. To that end, Medable recently partnered with several like-minded companies to launch the ACCESS initiative—short for American COVID-19 Collaborative Enabling Seamless Science. ACCESS is a master observational protocol that uses digital and mobile technologies to enable groundbreaking research. Our goal is to accelerate disease and immunity testing and clinical drug and vaccine development for COVID-19.
Powered by a mobile consumer application (see screenshot examples below), ACCESS is always available to hundreds of thousands of individuals in the U.S. whether they are home or not. It provides a digital onramp to diagnostics, monitoring, and clinical trials with an intensity of purpose around scientific discovery that can help people get back to work safely.
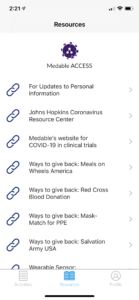
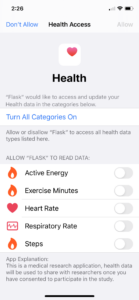

ACCESS makes it easy for individuals to contribute specific information about their COVID-19 experience, and then (with their permission) combine health records and wearable device data to enable rapid and significant discoveries. Participants can also opt in to learn more about current and future studies for diagnostics, treatments, and vaccines. The aggregated data provide researchers with a foundational framework for dynamic implementation of projects critical to help beat this disease.
The multi-company ACCESS initiative is led by Medable, together with technology, healthcare, and life sciences companies including BioIntelliSense, Datavant, Parexel, PWNHealth and the American Heart Association’s Center for Health Technology and Innovation. The infrastructure combines opt-in medical-grade wearable sensors, patient-reported data and outcomes, opt-in historical health data, and health record aggregation. Participants maintain control over their personal health data—and decide how they want to engage in potential studies.
As a master observational protocol, ACCESS accelerates research and clinical trials in the following important ways:
- Provides a ready-to-go, mobile primary protocol framework that can digitally harmonize essential clinical data across a diverse population of participants;
- Creates a foundational dataset for qualified researchers comprised of robust and validated medical data across a large participant population;
- Enables a pool of prequalified participants by using algorithmic matching to exclusion and inclusion criteria for sub-study clinical trials to reduce recruitment and enrollment timelines;
- Supports a flexible framework to accelerate participant digital consent and onboarding to sub-studies and clinical trials; and
- Removes barriers to participation and evidence generation through a decentralized clinical trial infrastructure that facilitates at-home research participation.
Treatment and Vaccine Development
One of the goals for ACCESS is to accelerate treatment and vaccine development by removing time-consuming barriers throughout the clinical drug development lifecycle (see Table 1). The strategy is to leverage the master protocol to collect critical baseline clinical trial data. These data are then rapidly used to derive research insights and establish a pool of prequalified or “trial-ready” participants for sub-study clinical trial recruitment. This participant-powered process is tied into best practices for informed consent and study transparency. If a participant is interested in a matched sub-study, the framework enables seamless consent, enrollment, and participation.
Table 1: Methods for Removing Time-Consuming Barriers to Drug Development
| Clinical Research Requirement | Accelerator |
| Baseline Data | ● Primary protocol collects robust baseline data to inform case and control datasets and provide natural history disease knowledge |
| Protocol Deployment | ● Streamlined framework for rapid sub-study protocol deployment that can be easily configured from within the master protocol to accommodate sub-study requirements |
| Recruitment | ● Prequalified population of individuals interested and actively participating in research |
| Enrollment | ● Data-driven prequalification enables activation of interested and eligible participant within 24 hours |
| Evidence Generation | ● Synthetic control data from master protocol data
● Potentially improved retention with decentralized/at-home trial format ● Data tokenization enables long-term follow-up and outcome data capture across medical record databases |
Real-World Data Collection and Vital Sign Monitoring
One challenging barrier to drug and vaccine development is the collection of real-world evidence for long-term outcome tracking. ACCESS reduces the barrier by enabling self-reported and biosensor data along with robust and validated real-world data capture across typically hard-to-consolidate data sources, including but not limited to prescription, medical record, lab, claims, and mortality data. Data may be collected for a 10-year duration to enable hard-to-measure long-term outcomes. Tokenization utilizes unique participant data elements to retrieve data from data repositories. Importantly, these data are only included from participants who actively consent for real-world data collection.
In our evaluation of research studies and clinical trials for diagnostic testing, treatment, and vaccine development with healthy participants or participants with mild to moderate COVID-19, the need to collect physiological data was common and important for active infection monitoring. Therefore, ACCESS enables real-time physiologic data collection across core vital signs using common consumer devices, including the Apple Watch. For research studies requiring continuous vital sign monitoring or U.S. Food and Drug Administration–cleared data collection, ACCESS currently provides the BioIntellisense BioSticker as a provisioned and integrated device and other devices, including AliveCor’s KardiaPro 6L for ECG monitoring and other biometrics.
Remote monitoring is also important to identify adverse reactions to therapies and vaccines. ACCESS provides an analytics framework to monitor data as well as remote nursing capabilities to monitor and manage participants. These aspects are critical to enabling at-home research, as few clinical trial teams can quickly provide at home in-person services when needed—and data volumes can often exhaust standard site and data management resources.
Contribution and Impact
ACCESS is a novel digital research framework that enables participants to power critical research from the comfort of their homes. The immediate purpose is to accelerate critical COVID-19 knowledge, research, and clinical drug and vaccine development. As a master observational protocol, it will allow partners to capture and combine information via pre-integrated digital technology—and rapidly launch sub-studies to accelerate research when time is of the essence.
We believe there is no going back to the “old” clinical trial structure. The new normal will be an improved, technology-enabled approach that is more efficient, decentralized, and patient-centric. Together, we can harness the power of the people to contribute to finding cures for disease, regardless of where they live.

Ingrid Oakley-Girvan, PhD, MPH, is Senior Vice President of Research and Strategy at Medable, a member of the Stanford Cancer Institute and the Canary Center at Stanford for Cancer Early Detection, and Director of the Data and Technology Proving Ground at the Public Health Institute.



