Clinical Researcher—January 2021 (Volume 35, Issue 1)
PRESCRIPTIONS FOR BUSINESS
Bernard Vrijens, PhD
Clinical researchers based in academic medical centers, private practice study sites, NGOs, or foundations know how crucial it is to monitor and manage patient medication adherence in a clinical study or research setting. According to the World Health Organization, up to half of patients with chronic diseases fail to take their medications properly.{1} Research shows many patients who are part of clinical trials do not maintain medication adherence either, despite being closely monitored.{2}
Historically, these protocol deviations have often gone undetected by the more traditional non-digital measures of adherence, such as pill counts, blood sampling, and patients’ self-reporting, as they inherently lack the precision to do so. Some of the most common culprits that prevent medication adherence from taking place include:
- Poor communication between the healthcare providers and patient.
- The patient having limited knowledge of the drug and how to use it.
- The patient’s fear of suffering adverse effects or side effects.
- The patient not agreeing that he or she needs the medication.
- The patient misunderstands complicated medication routines.
- The patient perceives no benefit from the medication.
- The patient forgets to take the medication.
Academic researchers work tirelessly to prepare the protocol of a study investigating medication adherence. Crucially, the method to measure medication adherence can highly impact the results and conclusions. Electronic monitoring of dosing history using a medication event monitoring system offers enhanced benefits. Electronic compilation of dosing history data, enabled through such systems by smart packages, is an effective way to monitor, identify, manage, and document the risks associated with poor patient adherence to medications in the research setting.
The Benefits
Digital medication adherence monitoring is straightforward and easy to implement in an academic clinical study without delays, and is applicable to all study participants with minimal burden for the patients. Patients are empowered as the solution is usable without any necessary configuration by patients. Such innovations are non-intrusive and friction free for patients. There is no need to combine an app or phone, nor to recharge/change the battery.
The analysis of medication adherence data collected using smart packaging enables researchers to:
- Quantify medication adherence and differentiate its three elements (initiation, implementation, and persistence) as suggested in the Medication Adherence Reporting Guidelines (EMERGE).{3}
- Assess the determinants of patient non-adherence to medications and determine the causal pathway between suboptimal drug exposure and outcomes.
- Manage medication adherence in individual patients by providing feedback on the patient’s drug dosing history, showing occurrences of errors that can jeopardize treatment outcomes.
Eliminating Bias in Results
Non-electronic methods like pill counts, blood sampling, and a subject’s self-reporting remain widely used, but do not allow researchers to distinguish between the three elements of medication adherence, tend to overestimate medication, and can thus bias the results of adherence research.
The following figure highlights how the different methods vary.
Figure 1: Measures Used to Help Monitor and Alleviate the Issue of Non-Adherence to Medications
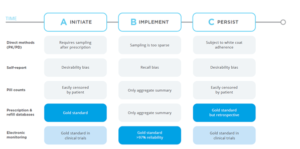
A Review of the Literature
A panel of international experts concluded that electronic monitoring is the optimal measurement approach for the detection of missed doses, extra doses, and wrong time intake.{4} Electronic monitoring is a robust indicator with error rates of <3% in research settings and clinical trials of when the patient took the prescribed dose of the drug.{5}
Compared to electronic monitoring, adherence is significantly overestimated using self-report, pill count, or healthcare provider rating. Those pre-electronic methods are sparse and biased, leading to sloppy estimates of medication adherence.{6} Based on records from ClinicalTrials.gov, between 2017 and 2019, 150 National Institutes of Health (NIH) research grants examined adherence to prescribed medications in which the combination of self-report and MEMS® Caps (a specific marketed type of medication event monitoring system) or other electronic monitoring system were the most common measurement approaches.
Medication Event Monitoring Systems (MEMS®) in Action
Many researchers are improving the power of their research and increasing the success of publication by adopting reliable state-of-the-art measurement systems of patient adherence to medications, as demonstrated in the following examples:
Example 1: Report of a MEMS® study led by the Ruedi Lüthy Foundation (formerly Swiss Aids Care International), Bern, Switzerland (2019, unpublished){7}
“According to the study results, MEMS® provides a better assessment of adherence levels when compared to the pill count method or self-report. Assessing adherence with MEMS® was a better predictor of viral load outcomes compared to use of pill counts. Clinicians should not use pill counts as the sole adherence assessment technique in adolescents as it is vulnerable to manipulation.”
“… pill count method, which is the most frequently practiced method to estimate adherence, was shown to grossly overestimate adherence. Since pill counts were discrepant to MEMS® results, we can assume that adolescents frequently dumped their pills so that they would appear as being adherent to their medication.”
“MEMS® had the widest distribution of adherence levels as compared to the pill count method. Only adherence measured by the MEMS® was significantly associated with the clinical outcomes of participants hence the MEMS® was shown to be a better predictor of adherence in adolescents on ART.”
Example 2: Feedback posted on ClinicalTrials.gov from a study coordinator who participated in a study led by the RAND Corporation in 2020
“[P]atients appreciate the MEMS® and love them to the maximum. Some of the patients were not willing to surrender the MEMS® back [because] they think without the MEMS®, their medication adherence will drop. …Almost all agree that [this type of medication event monitoring system] helps them to take their medication well in a sense that it will report them if they don’t take their medication…. …In most cases, MEMS® adherence moves in the same direction with someone’s health. Patients who show high MEMS® adherence also tend to have a low viral load. I am therefore confident that these solutions are really helpful to enable researchers do their work, but they are in themselves very helpful in motivating users to improve their medication uptake.”
Conclusions
Patient non-compliance with, or non-adherence to, medications is an important factor that can put the success of a clinical trial at risk. Medication event monitoring systems present a proven solution that is straightforward and easy to implement in any study. The method is applicable to all study participants without additional burden for the patient. In addition, it is a mature solution with track records in more than 1 million patients in research settings, including at more than 500 universities and research centers worldwide in more than 1,000 clinical trials.
In today’s research settings, solutions are needed that seamlessly measure and analyze patient medication adherence to support successful management of patient adherence to medications. Overlooking this risk can lead to significant issues; however, they can be easily diminished by implementing a mitigation plan based on proven digital medication adherence monitoring systems to maximize the chances of success to the study. Medication event monitoring systems have been a part of successful adherence research for more than 30 years, increasing the impact of academic research findings.
For more information on how medication adherence solutions can improve research and mitigate the effect of non-adherence, visit https://www.aardexgroup.com/services/academic/.
References
- https://www.who.int/chp/knowledge/publications/adherence_report/en/
- Blaschke TF, Osterberg L, Vrijens B, Urquhart J. 2012. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Ann Rev Pharmacol Toxicol 52:275–301.
- espacomp.eu
- Kronish IM, Thorpe CT, Voils CI. 2019. Measuring the multiple domains of medication nonadherence: findings from a Delphi survey of adherence experts. Transl Behav Med 3:ibz133. doi:10.1093/tbm/ibz133. Epub ahead of print. PMID:31580451.
- Vrijens B, Urquhart J. 2014. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther 95(6):617–26.
- El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. 2016. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol 82(1):268–79.
- https://www.ruedi-luethy-foundation.ch/en/home.html

Bernard Vrijens, PhD, is Scientific Lead for the AARDEX Group and an Invited Professor of Biostatistics at Liège University, Belgium.



