Clinical Researcher—March 2021 (Volume 35, Issue 2)
PEER REVIEWED
Erika Stevens, MA; Liz Wool, RN, BSN, FACRP, CCRA, CMT
As the number of global clinical trials continues to rise, so does the need and demand for qualified research support personnel, which further drive expectations for clearly established job functions. Variability in the assigned roles and responsibilities among clinical research coordinators (CRCs) creates opportunity to provide clarity in defining the profession.
This article identifies the gaps in industry recognition and classification practices for CRCs. Understanding national demographic benchmark trends among CRCs and clearly defining position expectations will provide insight into the professionalization of the CRC position. The ability to establish a clearly defined career roadmap for the CRC—one based on a thorough understanding of the role’s salient competencies—better enables job performance and provides opportunities for career advancement and credentials to those in the profession.
Background
The CRC (also referred to as clinical trial administrator, research coordinator, and other terms) role is not described or defined in regulations or in the Good Clinical Practice (GCP) E6 guideline of the International Conference on Harmonization.{1}
Although the field of clinical research continues to grow in the U.S., with the number of clinical trials having more than doubled in the past 10 years{2} (see Figure 1), much of the workforce supporting this growth remains unrecognized by the Bureau of Labor Statistics (BLS).{3}
Figure 1: Registered Trials on ClinicalTrials.gov, 2010–2020 (as of November 12, 2020)
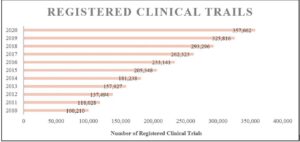
While absent data on CRCs, BLS published an article on occupations in biotechnology referencing CRCs, describing their primary functions as recruiting and screening patients who try new treatments and monitoring and reporting on patient progress.{4} As of 2019, medical scientists and clinical laboratory technologists/technicians are recognized and tracked in the annual occupational outlook handbook from the BLS, but absent still is a CRC listing. Medical scientists are defined as those who “conduct research dealing with the understanding of human diseases and the improvement of human health; engage in clinical investigation, research and development (R&D), or other related activities.”{5} Meanwhile, research managers, research analysts, and survey researchers make the list, but their definitions do not address the competencies required for the role of the CRC.
Arguably, understanding human diseases, improving health, and engaging in clinical investigation and R&D could fall under the purview of the CRC. While the BLS does not recognize CRCs, various membership-based organizations recognize clinical research personnel within the field of clinical research. For example, the membership of the Association of Clinical Research Professionals (ACRP) includes CRC as the largest specialty role represented in its ranks. Still, how does the occupation of the CRC become one that is recognized officially as a profession by regulatory authorities and other levels of government?
In Search of Professional Recognition
The first of four steps (see Figure 2) is to define the concept by aligning similar organizations into a common industry. Webster defines industry as, “manufacturing activity as a whole and [an activity] that employs a large personnel and capital especially in manufacturing.”{6} Similarly, the BLS defines industry as “a group of establishments that produce similar products or provide similar services.”{7} In this case, aligned organizations participating in clinical research would be classified as functioning within the clinical research industry.
Broadly defined, those who “engage in clinical investigation and R&D, or other related activities” are part of the clinical research industry—this includes executives, staff, and vendors tied to sponsors of studies (from pharmaceutical, medical device, biotech, and biologics firms, independent principal investigators acting as sponsors, patient recruitment specialists, contract research organizations [CROs], etc.), personnel at study sites (based in private healthcare practices, academic medical centers, health systems/hospital networks, site management organizations, etc.), and relevant employees in regulatory bodies (e.g., the U.S. Food and Drug Administration, Office for Human Research Protections, Centers for Medicare and Medicaid Services, etc.).
Figure 2: Steps for the Professionalization of CRCs
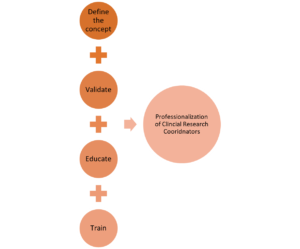
Thus, a wide swath of what may to the uninitiated seem to be only loosely related organizational occupations fall within the clinical research industry. The BLS allows for a given industry to have employees in dozens of occupations,{7} and leverages the North American Industry Classification System coding structure to group establishments together based on their primary activity and those with similar labor into 20 industry sectors.{8}
The next step in validating an occupation is to define the responsibilities directly related to the job role. In a presentation leveraging two national CRC datasets from the Clinical and Translational Science Award (CTSA) Research Coordinator Task Force, Speicher et al. present evidence of tasks well outside the original defined scope of clinical trial management.{9} Later, Speicher et al. published results of the CTSA’s CRC survey indicating the roles and responsibilities assigned to CRCs are vast and not clearly defined.{10} Many of the tasks identified in the results align with those defined by BLS as “participating in clinical research investigation.”
The defined competencies for the clinical research professional remained unclear until the Joint Task Force for Clinical Trial Competency published its competency domains for clinical research.{11} The task force outlined the knowledge and skills required throughout the clinical research enterprise and, in May 2018, ACRP published core competency guidelines for the CRC, identifying entry-level, mid-level, and senior-level competencies and tasks.{12} Competency models solidify required knowledge and mastery of tasks within an industry, providing detailed information about job requirements and proficiency.{13} Identifying and mapping the required skillset needed to perform the expected position enables assessment and confirmation of acceptable performance for the assigned job/role.
Many industries use education as a pathway for the levels of comprehension and ability necessary to perform job-based requirements. In the same career outlook article on jobs in biotechnology, BLS recognizes most CRC jobs require a minimum of a bachelor’s degree and in some positions a master’s degree.{14} A variety of academic programs offer industry-specific diplomas or degrees specializing in the field of work, and educational opportunities to support clinical research continue to expand. The Consortium of Academic Programs in Clinical Research (CoAPCR) lists 51 clinical research academic programs.{15} Leveraging the CRC competency model criteria, educational programs can more clearly align curricula to specified job functions.
A recent snapshot of the ACRP member database shows that 43.26% of respondents to a request about educational attainment hold a bachelor’s degree as their highest level of achievement, while 43.73% have one or more graduate degrees (see Table 1).{16}
Table 1: Reported Highest Level of Education of Responding ACRP Members in 2020
| Highest Education | Count | % of Total |
| High School Diploma | 259 | 2.64% |
| Associate/Two-Year Degree | 650 | 6.63% |
| Bachelor’s Degree | 4,260 | 43.26% |
| Master’s Degree | 3,095 | 31.57% |
| Doctorate Degree | 1,192 | 12.16% |
| Paraprofessional Diploma (LVN, medical assistant, etc.) | 347 | 3.54% |
| Total Respondents | 9,803 | 100% |
Following education, the pathway to professionalization often requires certification, licensing, or credentialing. Certification supports the mastery of a specific skillset that is aligned to the job. “A certification is a credential that you earn to show that you have specific skills or knowledge. They are usually tied to an occupation, technology, or industry. Certifications are usually offered by a professional organization or a company that specializes in a particular field or technology.”{17}
Professions requiring certifications/licensing are arrayed across many industries. In 2018, more than 48 million people reported that they hold an occupational license or certification.{18} While some employers require certification for clinical research positions, certification is not mandated throughout the industry. Still, data support increased trial performance with certification.{19}
Haeusler’s analysis of four retrospective multicentered trials combined ACRP’s principal investigator certification (CPI) and CRC certification (CCRC) as evidence of Good Clinical Practice training and reported significantly fewer protocol deviations among those certified.{20} Nearly 10 years later, Hodges and Akroyd’s study reported fewer protocol deviations among CPIs and suggested a requirement for principal investigator certification may improve data quality in clinical research.{21} Further, in a 2018 Drug Information Association meeting, Tufts, ACRP, and the WIRB-Copernicus Group presented data analyzing 7,000 active CRCs, finding those with ACRP certification have fewer protocol deviations compared to their non-certified peers.{22}
While the evidence supports improved clinical research performance with certification, we reiterate that neither the clinical research industry nor its regulators currently require certification. At any rate, ACRP’s exam-based CCRC program is accredited and has produced more than 20,500 certificants in its 28-year history.
Arguably, certification supports the pathway to professionalization for CRCs by virtue of being a data-validated measurement of CRC capability. An Association for Clinical and Translational Science assessment of training for CRCs identifies a gap in certification and recommends a formal assessment.{23} In a recent review of the literature, Bocchino et al. suggest that a blending of competency and performance outcomes may be required for assessing job performance.{24}
The roadmap to attain a BLS ranking for the CRC is well defined, and the research industry has collaborative work to do to achieve the goal of having the CRC recognized as a profession. Detailed in Table 2 are the requirements to be recognized as a profession by BLS.
Table 2: BLS Requirements and Clinical Trials Industry Status
| BLS Requirement | Status in Industry | Explanation |
| Pay | Not uniform in the industry.
Data available from ACRP. |
Median data for wage and salary workers. Includes the top 10% and bottom 10% of the workers in the occupation. |
| Typical Entry-Level Education | Not uniform in the industry. | What is required to enter the workforce for occupation. |
| Work Experience in Related Occupation | Not uniform in the industry. | Transferrable knowledge and skills. Common substitutes for formal types of training or education. |
| Other Experience | Not uniform in the industry. | Experience in volunteering or while in school that can aid in attaining the job. |
| Important Qualities | Not uniform in the industry. | Skills, aptitudes, and personal characteristics. |
| Certification, Licenses, Registrations | Not required to get a job as a CRC. | Are any of these needed for the occupation. If it is needed, how does the worker attain? |
| Work Environment and Workforce Schedules | Not uniform in the industry. | Working conditions, typical workplace, level of physical activity, working hours. |
| Work Performed | Detailed job descriptions are not uniform in the industry publication by Speicher et al.{10} | Responsibilities, duties, and tasks; who the CRC interacts with; and frequent technology used. |
| Training and On-the-Job Training Needed to Attain Competency |
Not uniform in the industry.
|
Post-employment classroom and on-the-job training needed for the occupation.
Internships and apprenticeships are addressed in this section for job training. Competencies published by ACRP, Society of Clinical Research Associates (SoCRA), and Multi-Regional Clinical Trials Center of Harvard. |
| Advancement | Not uniform in the industry. | What is required for advancement in the occupation (e.g., certification, formal education). Also, opportunities for advancement can come from within an organization (becoming a manager or supervisor, for example). |
| Number of Jobs | Needs to be compiled from various sources. | Employment, or size, of the occupation in the based year of the employment projections. |
| Job Outlook | Needs to be compiled from various sources. | Projected percentage change over a decade.
Job prospects for people to enter the occupation with information about how easy or hard it is to enter the occupation. |
| Employment Change | Needs to be compiled from various sources. | Projected numeric change in employment over a decade. |
| State and Area Data | Needs to be compiled from various sources. | Sources for employment, wages, and projections data by state and area. |
| Similar Occupations | Needs to be compiled from various sources. | Does another occupation have similar job duties or similar required skills? |
| More Information | Needs to be compiled from various sources. | Provides links to associations, organizations, and other institutions that provides readers with additional information. |
In order to define each of these areas in a uniform manner to be recognized as a profession by the BLS, the research industry needs to form a CRC Professionalization Workforce Alliance comprised of various professional associations (e.g., ACRP, SoCRA, Society for Clinical Research Sites) and sites (e.g., government, non-government, networks, etc.). This alliance would agree upon, promote, and implement the BLS requirements in order to demonstrate standardization of the CRC role in the research industry. This requires our industry to break down the “silos”—each stakeholder’s niche in the industry—for the greater good of having our CRCs recognized as professionals. This alliance would also provide the future framework and approach for the industry to collaborate on the professionalization of other roles, such as the site monitor/clinical research associate.
Summary
The clinical research industry is well positioned to align sites, sponsors, CROs, and other organizations supporting clinical research to clearly develop the roadmap for the professionalization of the CRC role. Leveraging the work of the Joint Task Force for Clinical Trial Competency and ACRP’s CRC core competencies defines the occupation. Industry-specific education and training provide the foundation for meeting the tasks assigned to the CRC role. Quantifying competency and confirming comprehension are garnered through assessment. The professionalization of CRCs relies on the culmination of these steps as referenced in Figure 2. As an industry, we are well positioned to implement the necessary training and to confirm the comprehension of defined competencies that will the catalyst for eventual BLS classification and recognition of CRCs as professionals.
References
- https://www.fda.gov/files/drugs/published/E6%28R2%29-Good-Clinical-Practice–Integrated-Addendum-to-ICH-E6%28R1%29.pdf
- https://clinicaltrials.gov/ct2/resources/trends
- https://www.bls.gov/ooh/a-z-index.htm#R
- https://www.bls.gov/careeroutlook/2002/fall/art03.pdf
- https://www.bls.gov/oes/current/oes191042.htm
- https://www.merriam-webster.com/dictionary/industry
- https://www.bls.gov/bls/glossary.htm#industry
- https://www.bls.gov/bls/naics.htm
- Speicher L, et al. 2010. Research Coordinator Task Force: The Research Coordinator Unplugged: What Should be on Their “Stop Doing List.” Presented at the 3rd Annual Clinical Research Management Workshop in Bethesda, Md.
- Speicher L, et al. 2012. The Critical Need for Academic Health Centers to Assess the Training, Support, and Career Development Requirements of Clinical Research Coordinators: Recommendations from the Clinical and Translational Science Award Research Coordinator Taskforce. Clinical Translational Science 5(6):470–5.
- Sonstein SA, et al. 2014. Moving from Compliance to Competency: A Harmonized Core Competency Framework for the Clinical Research Professional. Clinical Researcher 28(3):17–23. https://acrpnet.org/crjune2014/
- https://acrpnet.org/acrp-partners-in-workforce-advancement/entry-level-crc-competency-development-and-assessment-roadmap/
- https://skilledwork.org/publications/competency-based-credentials-case-studies/
- https://www.bls.gov/careeroutlook/2002/fall/art03.pdf
- https://coapcr.org
- ACRP membership database. 2020.
- https://www.careeronestop.org/FindTraining/Types/certifications.aspx
- https://www.bls.gov/opub/mlr/2019/article/professional-certifications-and-occupational-licenses.htm
- Redfearn S. 2018. Research Projects Show Credentialed Principal Investigators and CRCs Perform Better. CenterWatch Weekly 22(26).
- Haeusler JC. 2009. Certification in Good Clinical Practice and Clinical Trial Quality: A Retrospective Analysis of Protocol Adherence in Four Multicenter Trials in the USA. Clinical Research and Regulatory Affairs 26(1–2):20–3.
- Hodges A, Akroyd D. 2018. Does PI Certification Make a Difference? Applied Clinical Trials. https://www.appliedclinicaltrialsonline.com/view/does-pi-certification-make-difference
- Caruso S, et al. 2018. Quantifying the Impact of Credentialed Clinical Research Site Professionals on Clinical Trials Conduct Quality. Presented at the Annual Global Meeting of DIA in Boston, Mass.
- https://www.cambridge.org/core/services/aop-cambridge-core/content/view/81F737875036233E0978A1ECDAAB06F0/S2059866116000029a.pdf/div-class-title-education-and-training-of-clinical-and-translational-study-investigators-and-research-coordinators-a-competency-based-approach-div.pdf
- Bocchino JM, Butler J, Harper B. 2020. A Perspective on the Current State of Clinical Research Education and Training. Clinical Researcher 34(9):8–20. https://acrpnet.org/2020/11/10/a-perspective-on-the-current-state-of-clinical-research-education-and-training/

Erika Stevens, MA, is the 2021 Chair of the Association Board of Trustees for ACRP and leads Transformation Advisory Solutions for Recherche Transformation Rapide.

Liz Wool, RN, BSN, FACRP, CCRA, CMT, is President of Wool Consulting Group.



