Clinical Researcher—February 2023 (Volume 37, Issue 1)
OVER THE TRANSOM
Edited by Gary W. Cramer (gcramer@acrpnet.org), Managing Editor for ACRP
Another revolution around the sun has not only brought us all into 2023, but has also brought me to a head-on collision with my 17th anniversary of working for ACRP just as this issue of Clinical Researcher is about to go online. As Wallace Shawn’s character from The Princess Bride would say, I find this to be “Inconceivable!” Like a stranger in a strange land, I am boldly going into an undiscovered country, along with all the other sci-fi and fantasy references I can carry with me. You see, I’ve never worked this long for a single employer before (my previous record was 15 years and four months, to be precise, but somehow hitting my 16th year devoted to clinical research last year didn’t affect me as much as this year’s new notch in the belt); where do I go from here?
Well, I came onboard just in time for ACRP’s 30th anniversary in 2006 and was a seasoned pro at living the membership association lifestyle by the 40th in 2016, so typing away for another few years to see what the 50th anniversary celebration will look like shouldn’t be too much of an ask. In the meantime, revolutions of the “way things are” vs. “the way things should be” sort are happening everywhere you look and reach in the clinical research enterprise—in trial designs and technologies, in workforce training and development, in regulatory compliance, in data management, in patient recruitment and retention…the list goes on—as can be appreciated from the contents of this issue. Here are hints of some more revolutions you may wish to explore, brewing out there among organizations of note (no endorsements implied) in their various kingdoms and fiefdoms. Have fun storming the castle!…
Reaping Retention from Robust Employee Rewards
Employee turnover continues to plague the global market for talent in contract (or clinical, if you prefer) research organizations (CROs). The companies that will be successful in not only retaining top talent but also in recruiting will employ innovative approaches to their reward strategies, according to the latest Insights Report based on results from the recently published “BDO CRO Industry Global Compensation & Turnover Survey.” This Insights Report discusses how now, more than ever, companies are digging deep to find ways to make sure employees stay, which is stirring the buzz term “The Great Retention.”
Looking at compensation levels, plan design, and employee turnover, the annual survey collected data for 268 positions in the U.S. and 55 countries outside the U.S. to help clinical research outsourcing companies develop confidence in their pay levels by providing data necessary to gain insight into their compensation practices relative to the market.
AI and Big Data Impacts on Pharma Expected to Live Large in 2023
Artificial intelligence (AI) and Big Data will be the two most impactful technologies for the pharmaceutical industry for the fourth year in a row in 2023, according to a survey by GlobalData. The data and analytics company notes that 39% of healthcare industry professionals in the survey believed that AI would be the emerging technology bringing the greatest impact on the pharmaceutical industry in 2023, followed by Big Data with 27% of the selection.
The “State of the Biopharmaceutical Industry 2023” survey results reveal that AI and Big Data were trending as the two most disruptive emerging technologies since 2020, with a significant margin from the third choice in all four years (see figure below).
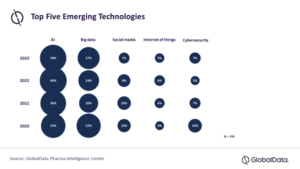
“It might take some time for AI and Big Data to display their true power, but the two technologies together are expected to play an important role in the industry, in terms of optimizing the entire pharmaceutical value chain,” said Elton Kwok, market research manager in Pharma at GlobalData. “This powerful duo can be applied to optimize a wide range of processes, from drug design to end-user reach.”
U.K. Independent Trial Sites Continue to Prosper Despite Decline of NHS Sites
The independent site management organizations (SMOs) which run clinical trials on behalf of the pharmaceutical industry continue to expand not only the number of trials being brought to the United Kingdom, but also the number of patients enrolled. While the Association of the British Pharmaceutical Industry reported that there was a substantial decline in patient numbers at National Health Service (NHS) sites in the U.K., independent SMOs are doing well.
According to Chris Dodd, chief commercial officer of Panthera Biopartners, “The SMOs which recruit tens of thousands of patients each year to clinical trial sites across the U.K. not only continued to run trials during the pandemic—including many of the major vaccine studies—but also rapidly recovered from COVID and are providing global pharmaceutical companies with access to more and more U.K. patients. However, the U.K. NHS sites have not only been closed to clinical trials for years, but are also very slow in moving from discussion to enrollment.”
Partnership Aims to Bolster Clinical Trials for Natural Products
United Natural Products Alliance (UNPA), an international trade association for natural products, and Radicle Science, a proof-as-a-service company offering a path for non-pharmaceutical products to clinically prove their true effects beyond placebo, have announced the launch of a partnership enabling UNPA members priority access to join in Radicle’s clinical trials as participants, furthering their synergistic missions. Collectively, they expect to generate important new findings to prove the effectiveness of natural products while illustrating the power of large-scale clinical data.
“For far too long, only patented pharmaceuticals could afford clinical trials, traditionally costing millions and taking years,” shared Dr. Jeff Chen, MD, cofounder and CEO of Radicle Science. “We’ve democratized access to clinical trials to close the proof gap between natural supplements and pharmaceuticals. As the UNPA has played such a monumental role in the legislative history of supplements, it’s fitting that UNPA members will help make history by personally participating” in the company’s clinical trials.
UNPA represents 100 natural products, dietary supplement, functional food, and scientific and technology and related service companies. Meanwhile, at the end of each Radicle study, volunteers are unblinded immediately and provided with personalized health reports so they can understand their product usage and the outcomes on their unique body.
▲▼▲



