Clinical Researcher—June 2024 (Volume 38, Issue 3)
OPINION
Elisa Cascade, MBA
Clinical research is thriving. A recent report suggests that the U.S. landscape for clinical trials will continue to grow by more than 5% over the next several years. The steady increase is driven by various factors, including the emergence of promising technologies and big data analytics that are creating opportunities to engage in and learn from potentially groundbreaking research.
While growth in clinical research development is expected to yield enormous human benefits, substantial growth can bring challenges, as well. For example, patient recruitment persists as one of the biggest obstacles to study success. Even as many healthcare providers supplement recruitment via data-driven patient identification and digital advertising, there is still a heavy reliance on site staff to establish a relationship with and qualify the patient referral for screening. At the same time, healthcare workforce levels are at an all-time low. For every one research coordinator seeking work in the U.S., there are seven jobs available and the ratio of clinical research nurses to potential positions is wider still, with only one nurse for every 10 positions.
In addition to the steady demand for patients and the site staff to screen them, research itself is more complex. Nuanced protocol designs for personalized medicines are adding heavy burdens, including stricter inclusion and exclusion criteria as well as federally mandated requirements for population representation. Biomarker-driven trials, which add both logistical and patient recruitment complications to trials, are becoming mainstream with half of all oncology trials and nearly 20% of all trials using biomarkers today. Further, the number of datapoints generated in Phase III trials is three times the data collected in late-stage trials a decade ago—at an average of 3.6 million datapoints.
Technology to the Rescue…Sort of?
To compensate for today’s mounting trial complexity, sponsors are turning to technology to improve efficiency and streamline workflows. Technology is having an important positive impact on trials—from accelerating the collection of higher quality data to driving more real-time data monitoring and much more. Yet, despite technology’s abundant benefits, the introduction of disparate point systems into clinical trial settings has resulted in unforeseen burdens on sites. The wide range of site- and sponsor-based systems has created a patchwork that can be difficult to navigate.
Based on feedback from Advarra’s 2023 Study Activation Survey Report, and validated through discussions with 10 experienced sites, a typical oncology study now uses about 22 different systems. For many studies, this number can be even higher with the use of wearable technologies, which are increasingly leveraged in diverse trial applications with more clinical certifications since the start of the pandemic. This massive proliferation of technology adds to site managers’ already substantial administrative burdens. In fact, nearly two-thirds of respondents in Advarra’s survey said that the burden caused by technology is greater than it was just five years ago (see Figure 1, which displays the various systems that a site would need to access in a sample oncology trial).
Figure 1: Technology Systems Accessed by Sites in an Oncology Clinical Trial
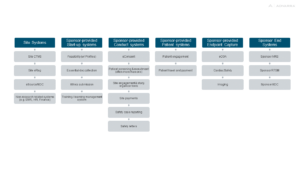
Source: Advarra discussions with 10 experienced oncology clinical trial sites representing a mix of academic medical centers, hospital systems, and site networks.
Interviewed site staff indicated that they have their own organization-wide systems (i.e., human resources, finance, electronic medical/health records), and technology-enabled sites would typically add three more tools such as a clinical trial management system (CTMS), electronic regulatory system, and electronic data capture (EDC) system. Beyond these site systems, sponsors often ask sites to use licenses of their systems for study start-up, study conduct, patient support, endpoints capture, plus some home-grown sponsor systems for randomization and supply chain management.
Given this web of technology, it is not surprising that set-up and training for sponsor-provided technology systems is now the most burdensome site activity, overtaking such traditionally arduous activities as contracting and budgeting. Unfortunately, there’s no slowdown in sight, as technology expands. Even as modern technologies have made a measurable impact on U.S. Food and Drug Administration inspection findings by reducing the number of protocol deviations and improving the overall quality of the research, sites are underwater. Worse, the more time site staff spend logging into and navigating disparate systems, the less time they have to work directly with patients and support vital patient recruitment and onboarding efforts.
Sign in Once, Access All
One solution to streamline the site staff’s experience is to enable single sign on (SSO) to all site technologies—site practice systems, site research applications, and sponsor-provided tools—through one set of login credentials. More than 80% of respondents in the 2023 Advarra site survey said that being able to use their own site credentials to log in to sponsor-provided systems was “extremely valuable” or “very valuable.”
Recognizing that the challenge of multiple systems and passwords extends more broadly than just sponsor-provided technology, it is not sufficient to centralize on a single vendor’s credentials. Fully streamlining the site’s experience across all their systems requires SSO capabilities that leverage the site’s own credentials. SSO frees site staff from managing and juggling dozens of passwords and increases focus on participant qualification and enrolled participant engagement.
“Large academic medical centers are conducting hundreds of trials for dozens of different sponsors, and this comes with a myriad of systems each with individual login credentials. Many staff require spreadsheets just to keep track of all their different passwords,” said Brian Sevier, PhD, chief operating officer at Yale Center for Clinical Investigation. “Not only does this complexity burden staff and slow trials, but it can also be a security issue such as when working with temporary consultants.”
SSO using an organization’s own credentials has the potential to impact security in several ways. First, it eliminates the need for maintaining a centralized spreadsheet tracker that would provide a threat actor with access to credentials to all site systems in a single location. SSO also enables the automated propagation of employee account deactivation across all site and sponsor systems used on a study at exit. Automated log-in deactivation saves time and reduces staff burden since this is typically a manual process, with a potential time lag. Further, there’s risk for user error in manual system deactivation, opening the site up to potential future security risk.
SSO is a critical step toward achieving a fully connected clinical trial ecosystem, but it’s also just the beginning. Sites can navigate this increasingly complex landscape by identifying the systems that offer the greatest benefit and integrating them seamlessly. Today, different processes and systems often require duplicative effort, making collaboration and real-time decision-making difficult for all stakeholders. In contrast, integrating systems via application programming interfaces (APIs) enables frictionless flow of data and documents across platforms, minimizing duplication of effort and providing a reliable, singular view of all study activities.
Sites, pharmaceutical companies, and technology providers all recognize the value of the connected ecosystem, however, achieving this goal will take time due to variances in the underlying data structures of individual systems. Working with technology providers that have multiple connected components and open APIs for integration with other site and sponsor systems should be prioritized when making technology purchase decisions.
Connect the Disconnect for a Better Future
Technology has had a transformative effect on clinical research but has also brought challenges. Site staff are using spreadsheet trackers to manage lists of systems and passwords across all their studies. Rather than reduce the number of systems, which would take the industry backwards, improve the technology. Implement SSO using a site’s own credentials to streamline the user experience while preserving important technology advantages in areas like data capture, study compliance, information transparency, and more.
Technology is here to stay, so the focus needs to remain on streamlining the site experience. The industry already has the technical capability to align on a single standard for site access to clinical research systems, and that standard should leverage their own credentials, not yet another externally created identity. With more systems coming online regularly, the time to act is now. Without properly corralling and managing this growing ecosystem, we stand to curb clinical research’s promising growth.

Elisa Cascade, MBA, (elisa.cascade@advarra.com) is Advarra’s Chief Product Officer, responsible for driving the company’s product vision and management product design and development. She brings more than 30 years of experience in the clinical research industry with a focus on using technology to transform clinical research for all stakeholders. She also serves as the 2024 Chair of the Board of Trustees for the Association of Clinical Research Professionals.



