Clinical Researcher—April 2025 (Volume 39, Issue 2)
PEER REVIEWED
Viswakanth Makutam, PharmD, MS, ACRP-CP; Marjan Doostan, PharmD, MS, BCGP
Despite advancements in oncology, clinical trials for blood cancer face significant challenges in achieving subject diversity. The following systematic review and meta-analysis estimate the extent of these challenges and their implications for clinical outcomes. Data from recent trials are analyzed to identify demographic, genetic, and clinical characteristics, highlighting gaps and proposing strategies for improvement.
Key findings indicate substantial underrepresentation of certain groups, particularly racial and ethnic minorities. This lack of diversity can result in disparities in research findings and treatment outcomes, with lower-income individuals and minority communities facing additional socioeconomic and logistical barriers to participation. Historical abuses have fostered mistrust in the healthcare system, further reducing enrollments of minority groups.
Data from ClinicalTrials.gov reveal a persistent lack of racial diversity in blood cancer trials, particularly in leukemia, where White participants dominate. The COVID-19 pandemic has brought renewed attention to these issues, though improvements remain marginal.
Meta-analysis findings show that trials with greater diversity report more comprehensive safety and efficacy data, underscoring the importance of inclusive research. We offer our perspectives based on insights gained from this effort in the hope of guiding the development of more effective and equitable treatment strategies for patients with blood cancers. Proposed strategies to address these challenges involve mandatory reporting of demographic data, stronger community outreach, and financial/logistical support to improve participation.
Background
Clinical trials are essential for advancing treatment options for patients with blood cancers such as leukemia. These trials are the cornerstone of evidence-based medicine, providing insights into the safety and efficacy of new therapies. However, one longstanding challenge is the lack of diversity among trial participants. This shortfall can lead to skewed results and limit the generalizability of findings, potentially compromising the efficacy and safety of treatments across different demographic groups.{1}
Ensuring participant diversity is crucial for multiple reasons. First, genetic variations across racial and ethnic groups can influence how patients metabolize medications and respond to treatments. Certain genetic markers present in specific populations may affect drug efficacy and the risk of adverse events. Therefore, treatments that appear effective in a homogeneous trial population may not perform as well in a diverse, real-world setting.{2}
Several studies underscore the importance of diversity in clinical trials. One such study demonstrated that including a broad range of racial and ethnic groups can lead to more accurate assessments of drug efficacy and safety, ultimately resulting in better health outcomes for all patients.{3}
Despite these recognized benefits, achieving adequate representation remains difficult. Barriers include socioeconomic factors, cultural differences, lingering mistrust of the healthcare system, and logistical issues that prevent participation by underrepresented groups. Overcoming these barriers is essential to ensure that findings are truly applicable to all patient populations, thereby improving overall medical research and treatment outcomes.{2}
Methods
Search Strategy and Study Selection
To comprehensively assess the state of diversity in blood cancer clinical trials, we conducted a systematic search of multiple databases, primarily focusing on ClinicalTrials.gov. Additional databases, including PubMed, Embase, and the Cochrane Central Register of Controlled Trials, were also examined to ensure thorough review. The search strategy included terms such as “leukemia,” “carcinoma,” “blood cancer,” “clinical trial,” “diversity,” “race,” and “ethnicity.” The search was limited to studies published from 2000 to 2023 to capture recent trends and developments in clinical trial diversity.
Inclusion Criteria
Studies were included if they met the following criteria: the primary focus had to be on blood cancer, particularly leukemia and carcinoma; trials were required to report demographic data, including the ethical and ethnic composition of the study population; studies needed to address clinical issues related to the efficacy and safety of the treatment under investigation; only completed trials with available results were included to ensure the availability of outcome data; and papers had to be published in English to facilitate analysis and interpretation.
Exclusion Criteria
Studies were excluded based on the following criteria: trials that did not provide detailed demographic information on participants were excluded; studies with incomplete or inconclusive clinical outcome data were not considered; if multiple publications from the same study existed, only the most comprehensive and recent report was included; and trials involving animal models or in vitro studies were excluded to focus on human clinical trial data.
Data Extraction and Analysis
Data were independently extracted by two reviewers using a standardized data collection form. Extracted data included trial identifiers (e.g., National Clinical Trial [NCT] number), demographics (age, gender, race), genetic information (if available), clinical outcomes (efficacy, adverse events, overall survival), and trial design and methodology.
Statistical Methods
A meta-analysis was conducted to integrate findings from the included studies. Fixed-effects and random-effects models were employed. The fixed-effects model was used for homogeneous studies, where variability was primarily due to within-study error rather than differences between studies. This model calculates a weighted average of the study-specific estimates, with weights inversely proportional to their variances. The random-effects model was applied when there was significant heterogeneity among the studies. This model accounts for both within-study and between-study variability, assuming that study-specific estimates vary not only due to sampling error but also due to real differences in effect sizes across studies.
Heterogeneity among studies was assessed using the I² statistic, which measures the proportion of total variation due to actual differences rather than chance. An I² value of 0 indicates no heterogeneity, while higher values suggest greater variability. The Chi-square (Q) test was also used to detect heterogeneity. To assess publication bias, funnel plots were generated, and an Egger’s test was performed. Funnel plot asymmetry or a significant Egger’s test result indicate potential publication bias.
Where data permitted, subgroup analyses were conducted to assess differences in treatment outcomes across various demographic groups, such as race and ethnicity. This helped identify potential disparities and areas requiring further investigation. Data analysis was performed using statistical software, including R (meta and metafor packages) and Stata, ensuring robust and reproducible results.
Results
Demographic Disparities
A key issue in blood cancer clinical trials is the underrepresentation of certain demographic groups. For example, the Pediatric Acute Leukemia (PedAL) Screening Trial (NCT04726241), targeting children with relapsed or hard-to-treat leukemia, lacks comprehensive data on the racial and ethnic composition of its participants. This gap makes it difficult to assess inclusivity and generalize findings to diverse populations. Table 1 presents the racial and ethnic distribution of participants in various blood cancer trials, highlighting disparities in representation.
Table 1: Racial and Ethnic Distribution of Participants in Selected Blood Cancer Clinical Trials{4-13}
| Clinical
Trials. gov ID |
N | White | Hispanic/Latino | American
Indian/ Alaska Native |
Asian | Black/
African American |
More Than One Race | Unknown/
Unreported |
|||||||||
| NCT046 29443{4} | 17 | 14 | 3 | 0 | 0 | 0 | 0 | 0 | |||||||||
| NCT041 45531{5} | 228 | 158 | 3 | 5 | 10 | 24 | 2 | 29 | |||||||||
| NCT036 77596{6} | 38 | 34 | 0 | 3 | 0 | 0 | 0 | 1 | |||||||||
| NCT040 03012{7} | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| NCT038 73493{8} | 14 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| NCT046 25413{9} | 15 | 14 | 1 | 0 | 0 | 0 | 0 | 0 | |||||||||
| NCT043 37138{10} | 32 | 26 | 0 | 0 | 0 | 2 | 0 | 4 | |||||||||
| NCT043 84848{11} | 94 | 81 | 0 | 0 | 1 | 1 | 0 | 11 | |||||||||
| NCT040 55844{12} | 14 | 12 | 0 | 0 | 0 | 1 | 0 | 1 | |||||||||
| NCT039 41964{13} | 60 | 55 | 0 | 6 | 5 | 11 | 2 | 46 | |||||||||
| Total | 522 | 418 | 7 | 14 | 16 | 39 | 4 | 92 | |||||||||
| Total
% |
80.1 | 1.1 | 1.0 | 2.1 | 6.3 | 0.4 | 8.8 | ||||||||||
Genetic Representation
Genetic diversity in clinical trials is crucial for understanding disease etiology and treatment responses. The European Rare Blood Diseases Platform (ENROL) (NCT06250595) aims to standardize and integrate data from various registries on rare hematological conditions across Europe, emphasizing the importance of genetic diversity. ENROL seeks to address data fragmentation and promote more inclusive research by mapping European Union–wide demographics and genetic information. Table 2 summarizes genetic representation across selected trials, highlighting variability in data collection and potential gaps in representation.
Table 2: Summary of Genetic Representation Across Selected Blood Cancer Clinical Trials{4-13}
| ClinicalTrials.gov ID | Genetic Data Available | Genetic Variability Reported |
| NCT04629443{4} | Yes | Limited |
| NCT04145531{5} | Yes | Moderate |
| NCT03677596{6} | No | Not Applicable |
| NCT04003012{7} | No | Not Applicable |
| NCT03873493{8} | Yes | Limited |
| NCT04625413{9} | No | Limited |
| NCT04337138{10} | Yes | Not Applicable |
| NCT04384848{11} | Yes | Moderate |
| NCT04055844{12} | No | High |
| NCT03941964{13} | Yes | High |
Clinical Outcomes
The Resiliency in Aged Adults Undergoing Bone Marrow Transplant (ANSWER) trial (NCT04188678) at Johns Hopkins focuses on patients over 60, a demographic often underrepresented in clinical trials. The study aims to identify factors contributing to better post-transplant outcomes. Early data suggest that comprehensive geriatric assessments may improve patient counseling and treatment planning. By addressing age-related differences in clinical outcomes, this trial provides valuable insights for developing tailored treatment strategies for older adults.
Innovative Approaches
The Multi-Institutional Prospective Exploration of Expanded Multi-Antigen Specifically Targeted Lymphocytes (RESOLVE) trial (NCT02203903) is investigating the use of tumor-specific cytotoxic T lymphocytes in treating high-risk hematologic malignancies. This trial includes diverse patient groups, such as those with acute leukemia, carcinoma, and myelodysplastic syndrome, to assess the safety and efficacy of this novel therapy. By incorporating a diverse patient population, the RESOLVE trial aims to enhance the generalizability of its results.
These examples highlight the importance of addressing demographic and genetic diversity in clinical trials. Ensuring diverse representation can lead to more accurate and generalizable findings, ultimately improving patient outcomes across different populations.
Challenges and Gaps
Despite efforts to increase diversity in clinical trials, challenges remain. For example, the Phase I Study of Tremelimumab, Durvalumab, High-Dose Chemotherapy, and Autologous Stem Cell Transplant (NCT02716805) faced regulatory hurdles and safety concerns, leading to a partial clinical hold and limited participant diversity. Its complexity and strict inclusion criteria further restricted broad patient representation.
Literature Review
Causes of Diversity Challenges and Impact
A comprehensive literature review reveals several factors contributing to the lack of diversity in clinical trials. One significant barrier is socioeconomic inequality. Lower-income individuals often lack essential resources such as transportation, childcare, and the ability to take time off work, making participation difficult. Another critical issue is the lack of awareness among many minority groups about clinical trial opportunities and the importance of their participation. This knowledge gap limits trial diversity. Additionally, historical abuses, such as those committed during the Tuskegee Syphilis Study, have fostered deep-seated distrust in the healthcare system among minority groups, further discouraging participation. Logistical challenges also play a role; practical barriers, such as trial site locations and inflexible scheduling, can prevent otherwise willing individuals from enrolling.
The impact of these diversity challenges is profound. Treatment outcomes may vary, with some therapies being less effective or causing different side effects in underrepresented populations. The lack of diverse data also limits understanding of treatment effectiveness across populations. A review of ClinicalTrials.gov data highlights this issue, showing a persistent lack of racial and ethnic diversity in many clinical trials. For instance, selected leukemia trials have a majority of White participants, with very low representation from Hispanic/Latino, Black/African American, Asian, and other minority groups. This disparity underscores the need for more inclusive clinical trial practices to ensure broader applicability of findings.
The data clearly show a predominant representation of White participants, with minimal inclusion of other racial and ethnic groups. Such disparities limit understanding of treatment responses across diverse populations, potentially resulting in less effective or even harmful outcomes for underrepresented groups.
Changes in Diversity of Trials Before and After COVID-19
The COVID-19 pandemic has heightened awareness of the lack of diversity in clinical trials. Pre-pandemic data already indicated significant underrepresentation, and this trend persisted during the pandemic despite increased efforts to address these disparities. However, significant gaps in representation remain (see Table 3).
Table 3: Comparison of Pre- and Post-COVID-19 Clinical Trial Diversity{14-15}
| Time Period | White | Hisp/Lt. Amer. | AmInd/Alaska Native | Asian | Black/Af. Amer. | More Than One Race | Unknown/
Unreported |
| Pre-COVID | 78% | 2% | 1% | 3% | 5% | 1% | 10% |
| Post- COVID | 75% | 3% | 1% | 4% | 7% | 2% | 8% |
The data suggest a slight improvement in diversity post-COVID-19, with marginal increases in the representation of Hispanic/Latino, Asian, Black/African American, and multiracial participants. However, these changes are modest, indicating that the pandemic has led only to incremental progress. The persistent lack of significant diversity underscores the ongoing need for more robust and systemic changes to ensure greater representation in clinical trials. The slight improvements observed can be attributed to increased awareness and initiatives aimed at enhancing trial inclusivity during the pandemic. However, the data highlight that these efforts alone are insufficient. More comprehensive strategies are needed to address the deeply entrenched barriers to participation faced by minority groups.
Efforts to increase diversity must include targeted outreach and education campaigns, along with initiatives to build trust within communities historically marginalized in medical research. Also, practical support, such as providing transportation, childcare, and compensation for time off work, can help alleviate socioeconomic barriers that hinder participation.
Meta-Analysis
A comprehensive meta-analysis of blood cancer clinical trials underscores the importance of diversity in research. Trials with higher participant diversity report more comprehensive safety and efficacy data, highlighting the need for inclusive research practices to ensure broadly applicable outcomes. The meta-analysis incorporated data from multiple regions, enabling a thorough comparison of trials with varying levels of diversity. This approach provided a clear understanding of the impact of participant diversity on clinical outcomes. Trials with diverse populations reported fewer adverse events and demonstrated more robust efficacy across different demographic groups. These findings suggest that treatments tested in diverse trials are more likely to be safe and effective for a broader patient population.
The analysis also showed that inclusive research practices produce more reliable and generalizable results. A lack of diversity in clinical trials can lead to findings that fail to represent all patient groups, potentially resulting in less effective or harmful treatments for underrepresented populations. Ensuring diversity is essential for developing truly effective therapies.
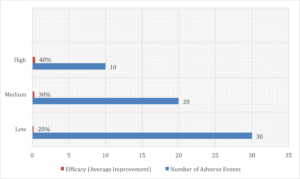
The meta-analysis data make a strong case for systematically including diverse populations in clinical trials. Figure 1 illustrates the impact of participant diversity on clinical outcomes, showing that more diverse trials report fewer adverse events and stronger efficacy, reinforcing that inclusive research is both ethical and scientifically beneficial.
Figure 1: Impact of Participant Diversity (High, Medium, Low) on Clinical Outcomes
Proposed Solutions
Building on the literature review, several solutions are proposed to address diversity challenges in clinical trials:
- First, mandatory reporting should be implemented to require all trials to disclose detailed demographic data, ensuring transparency and better diversity monitoring.
- Second, increasing community engagement through outreach and education in minority communities can raise awareness of clinical trial opportunities and the importance of participation.
- Third, incentivizing participation by providing financial and logistical support—such as transportation, childcare, and paid time off—can help remove barriers for underrepresented groups.
- Finally, designing flexible trials that accommodate the needs of diverse populations can encourage broader participation.
These strategies aim to tackle the root causes of diversity challenges in clinical trials, fostering more inclusive and effective research.
Conclusion
Achieving diversity in blood cancer clinical trials is not just a goal but a necessity for advancing oncology research and ensuring equitable patient care. Including diverse populations in trials makes findings more applicable across all demographic groups, leading to treatments effective across racial, ethnic, and socioeconomic backgrounds.
Despite persistent challenges—such as socioeconomic barriers, lack of awareness, and historical distrust in the healthcare system—there are clear pathways to overcoming these obstacles. Implementing targeted strategies and systemic changes is essential to breaking down these barriers.
Mandatory reporting of detailed demographic data can provide a clearer picture of disparities in clinical trials and help develop strategies to address them. Increasing community engagement through outreach and education can raise awareness of clinical trials’ importance and encourage participation from underrepresented groups. Financial and logistical support for participants can alleviate practical challenges that prevent many from enrolling. Likewise, designing flexible and inclusive clinical trials that accommodate diverse participant needs can further improve accessibility and representation.
By fostering inclusive research practices, the scientific community can ensure that medical advancements benefit all populations equitably. This not only improves overall patient care but also strengthens the robustness and generalizability of research findings. Diverse clinical trials provide a deeper understanding of how different populations respond to treatments, informing the development of more effective and safer therapeutic interventions.
References
- Daver N, Cortes J, Kantarjian H, Ravandi F. 2016. Acute myeloid leukemia: advancing clinical trials and promising therapeutics. Expert Rev Hematol 9(5):433–45. doi:10.1586/17474086.2016.1158096. PMID:26910051. PMCID:PMC5006674.
- Bibbins-Domingo K, Helman A. 2022. Why Diverse Representation in Clinical Research Matters and the Current State of Representation within the Clinical Research Ecosystem. National Academies Press (U.S.). https://www.ncbi.nlm.nih.gov/books/NBK584396/
- Vidal L, Z. Dlamini, Qian S, Rishi P, Karmo M, Joglekar N, et al. 2024. Equitable inclusion of diverse populations in oncology clinical trials: deterrents and drivers. ESMO Open 9(5):103373. https://pmc.ncbi.nlm.nih.gov/articles/PMC11090874/
- Phase I/II Trial of S64315 Plus Azacitidine in Acute Myeloid Leukaemia. https://clinicaltrials.servier.com/wp-content/uploads/CL1-64315-004-laysummary-2014.02.21.en_.pdf
- ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT04145531
- Özcan M, Cassaday RD, Singh P, Zarzycka E, Zhang X, Nègre E, et al. 2021. The Efficacy and Safety of Low-Dose Inotuzumab Ozogamicin in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia: Interim Results of a Phase 4 Study. Blood 138(Supplement 1):1208.
- ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT04003012
- Clinical Trial: NCT03873493 – My Cancer Genome. 2018. https://www.mycancergenome.org/content/clinical_trials/NCT03873493/
- ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT04625413
- ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT04337138
- ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT04384848
- ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT04055844
- ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT03941964
- U.S. Food and Drug Administration. 2020. Drug Trials Snapshots Summary Report: 2015–2019. Center for Drug Evaluation and Research. https://www.fda.gov/media/143592/download
- U.S. Food and Drug Administration. 2023. Drug Trials Snapshots – Annual Reports and Demographic Data. Center for Drug Evaluation and Research. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots

Viswakanth Makutam, PharmD, MS, ACRP-CP, is a Clinical Research Quality Associate and volunteer peer reviewer for ACRP’s Clinical Researcher journal.

Marjan Doostan, PharmD, MS, BCGP, is a Clinical Research Coordinator in St. Paul, Minn. A licensed Australian pharmacist, she was inspired by her frontline work during the COVID-19 pandemic to complete the Master of Science in Applied Clinical Research program at St. Cloud State University. She brings a strong foundation in geriatric pharmacy and a commitment to advancing evidence-based clinical trials.



