Clinical Researcher—April 2025 (Volume 39, Issue 2)
SITES & SPONSORS
Alen Hadzic, MIE; LaShoun Sanders, BS; Justin Scott Brathwaite, MBA
The Implications of Clinical Trial Contract and Budget Delays: By the Numbers
Clinical trial contract and budget negotiations have become increasingly complex in recent years, placing significant financial strain on clinical research sites. For example, the average time to negotiate a contract now exceeds 100 days.{1} For contract research organizations (CROs), sponsors, and sites, these negotiations have become a major bottleneck delaying study startup and site activation, while also negatively impacting recruitment and study progress.
Aside from an increase in the overall complexity of the contracts and budgets themselves, sites also face frequent payment delays—often waiting up to 90 days to receive compensation.{2} These factors place immense pressure on sites, making day-to-day operations difficult, since 80% of sites have less than six months of available operating cash on hand.{3}
The cumulative effect of these financial delays can be severe. A survey by the Clinical Trials Transformation Initiative revealed that nearly 40% of sites reported ceasing participation in clinical trials due to payment delays.{4} Similarly, an earlier 2017 survey from the Society for Clinical Research Sites showed that 20% of sites had reported net profits of less than 5%.{5}
These financial constraints highlight the growing challenges faced by research sites in managing the increasing costs associated with clinical trials. Without timely payments and fair compensation, sites risk struggling to stay financially viable, ultimately hindering the progress of clinical research.
Contract Negotiations and How to Flex Your High-Performance Site Muscles: Key Strategies from the Nation’s Top Site Management Organizations
High-performance clinical research sites must leverage strategic negotiation techniques to maximize accurate representation of site value, streamline operations, and enhance revenue opportunities. Contract negotiations are a fundamental aspect of clinical trial agreements, directly influencing site profitability, operational efficiency, and long-term partnerships with sponsors and CROs. To secure favorable contract terms, top site management organizations (SMOs) emphasize that research sites must not only meet regulatory and operational benchmarks, but also actively demonstrate their value proposition.{6}
Industry-leading SMOs nationwide have developed structured approaches to negotiations, ensuring that their sites are prioritized in trial selection and well-compensated for their contributions. According to top-performing SMOs, research sites must always implement the following six key contract negotiation strategies to achieve optimal outcomes:
- Always Use Data-Driven Justification of Site Performance
Top SMOs stress that research sites must always utilize historical site performance metrics, including patient enrollment rates, protocol compliance, and retention statistics, to justify higher per-patient payments and streamlined budget allocations. Presenting concrete data on enrollment speed and diversity compliance ensures that contracts recognize high efficiency and a lower risk profile.{6,7} - Always Leverage Physician-Embedded Research Models
Unlike traditional standalone research sites, leading SMOs emphasize the importance of integrating research within private medical practices.{8} This allows for rapid patient identification and streamlined study execution. Sites must always highlight this model in negotiations to demonstrate that enrollment efforts are diversified. This helps to increase study success rates, positioning sites preferred choices for sponsors seeking efficiency and diversity.{9} - Always Prioritize Site Selection and Budgeting
Top SMOs recommend that research sites must negotiate contracts that establish them as preferred providers, ensuring first access to new study opportunities.{9} Sites should always align with CROs and sponsors on multi-site agreements to secure recurring revenue streams while reducing the competitive burden of single-site contract awards. - Always Mitigate Risk and Ensure Compliance Excellence
Sponsors seek sites that minimize operational risks, including protocol deviations and patient noncompliance. Leading SMOs advise that sites must always reinforce their commitment to regulatory adherence and risk mitigation strategies.{10} Contract terms should include performance-based incentives and flexible timelines that benefit both the site and the sponsor. - Always Incorporate Advanced Technology and Decentralized Trial Capabilities
Research sites should seek to integrate artificial intelligence–driven patient recruitment, remote quality assurance monitoring, and eSource documentation to strengthen their bargaining power in contract negotiations. Top SMOs insist that sites demonstrate cost saving, efficiency improvements, and overall forward-thinking, to ensure contracts with higher reimbursement rates and extended study timelines.{11} - Always Establish Strategic Partnerships in Digital and Online Enrollment
Having a trusted partner in digital and online enrollment services with a proven methodology that provides reliable enrollment timelines is a critical negotiation factor. Top SMOs highlight that research sites must always demonstrate their investment in innovative enrollment methods to enhance study feasibility and execution.{11} Sponsors and CROs prioritize sites that proactively adopt new strategies and forward-looking solutions to optimize performance.
The Impact of Fast Enrollment on Budget Negotiations
It all boils down to how sponsors and CROs prioritize efficiency, and patient enrollment is often the biggest bottleneck in clinical trials. Sponsors and CROs prioritize sites that enroll, not recruit, patients quickly, as enrollment is the most critical and time-sensitive aspect of a clinical trial; it is important to note the difference.
A site with a proven track record of fast enrollment becomes highly desirable, enabling it to demand fairer budgets upfront. Sites that can guarantee rapid enrollment can negotiate for better financial terms, as accelerating enrollment timelines helps keep studies on track, reduce delays, and minimize costs linked to prolonged enrollment.
Fast enrollment also improves cash flow by enabling milestone payments to be reached sooner. Many trials structure payments around key milestones, such as completing enrollment or specific patient visits. Sites that enroll patients quickly can access these payments faster, reducing financial strain. In some cases, sponsors may adjust budgets mid-study for high-performing sites that exceed expectations, recognizing their role in keeping the trial on schedule.
Leveraging Social Media for Fast Enrollment
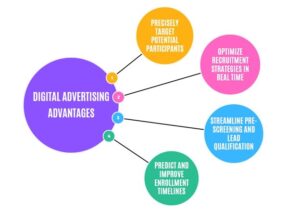
One effective way to achieve fast enrollment is through targeted social media and other modes of digital advertising. Traditional recruitment methods often struggle to reach diverse patient populations, while digital advertising provides direct engagement with potential participants with precision. As illustrated in Figure 1, social media platforms enable research sites to:
- Precisely Target Potential Participants—Utilize demographic filters such as age, gender, and location through radial targeting to reach the most relevant audience.
- Optimize Recruitment Strategies in Real Time—Test multiple targeting approaches simultaneously and adjust budgets based on performance metrics to maximize cost-effectiveness.
- Streamline Pre-Screening and Lead Qualification—Use lead-generation ads with conditional questionnaires based on inclusion and exclusion criteria in protocols to filter and register only qualified participants, reducing enrollment inefficiencies and costs.
- Predict and Improve Enrollment Timelines—Leverage real-time campaign data to estimate enrollment completion and refine strategies for faster, more cost-effective enrollment efforts.
Figure 1: Digital Advertising Advantages
Once a targeted digital campaign is launched, real-time metrics can predict enrollment timelines by tracking ad performance and pre-screening progress. As potential patients (leads) register and pre-screening is completed, the cost per lead decreases. This is because social media platforms use native algorithms that refine targeting based on previously qualified leads, reducing overall costs as each new qualified lead is registered and increasing campaign performance, resulting in a greater amount of qualified leads for the same budget.{12}
Fast enrollment offers research sites a strategic advantage in overcoming underfunded budgets in clinical trials.{13,14} By leveraging social media-driven enrolment strategies, sites can accelerate enrollment, secure better financial terms, and ensure long-term sustainability. As the industry evolves, adopting digital outreach will be crucial for maintaining competitiveness and financial viability in clinical trials.
High-performance research sites must always approach contract negotiations with strategic foresight, leveraging data, operational excellence, and technological advancements to maximize their value proposition. The insights from top SMOs nationwide demonstrate that a well-structured negotiation framework leads to stronger sponsor partnerships, optimized revenue streams, and increased site prioritization in clinical trials. By consistently applying these key strategies, high-performing sites can drive financial sustainability and research innovation.
References
- Lawrence CE, Bruce VNM, Salberg LD, Edwards T, Morales C, Palm M, Bernard GR. 2023. Quantitative assessment of the impact of standard agreement templates on multisite clinical trial start-up time. Journal of Clinical and Translational Science 7(1):e204. https://doi.org/10.1017/cts.2023.622
- Society for Clinical Research Sites. 2023. The 2023 Site Landscape Survey. https://myscrs.org/download/2023-site-landscape-survey-white-paper/?tmstv=1732045361
- Garton W. 2024. Elevating clinical research site relationships with new budget and payment paradigms. Applied Clinical Trials. https://www.appliedclinicaltrialsonline.com/view/clinical-research-site-relationships-budget-payment-paradigms
- Fassbender M. 2016. Payment tech automates reimbursements to clinical trial sites. Outsourcing-Pharma.com. https://www.outsourcing-pharma.com/Article/2016/07/20/Payment-tech-automates-reimbursements-to-clinical-trial-sites
- Society for Clinical Research Sites. 2018. Site Landscape Survey. https://www.outsourcing-pharma.com/Article/2018/12/19/A-year-in-review-A-perspective-from-the-Society-for-Clinical-Research-Sites
- Harper B, Smith Z. 2021. Characterizing Clinical Data Management Challenges and Their Impact. (Tufts Center for the Study of Drug Development/IBM Watson Health benchmarking study.) Applied Clinical Trials. https://www.appliedclinicaltrialsonline.com/view/characterizing-clinical-data-management-challenges-and-their-impact
- Senn C. 2025. Budgeting and contracting best practices for research sites. (Sponsored blog for Association of Clinical Research Professionals from Advarra). https://acrpnet.org/2025/03/17/budgeting-and-contracting-best-practices-for-research-sites
- Mayer Brown LLP. 2024. Key takeaways from clinical trial site management organizations (SMOs). https://www.mwe.com/insights/key-takeaways-clinical-trial-site-management-organizations-smos/
- U.S. Food and Drug Administration. 2025. Diversity action plans to improve enrollment of participants from underrepresented populations in clinical studies. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-action-plans-improve-enrollment-participants-underrepresented-populations-clinical-studies
- Barnes B, Stansbury N, Brown D, Garson L, Gerard G, Piccoli N, Jendrasek D, May N, Castillo V, Adelfio A, Ramirez N, McSweeney A, Berlien R, Butler PJ. 2021. Risk-based monitoring in clinical trials: Past, present, and future. Therapeutic Innovation & Regulatory Science 55(1):91–8. https://doi.org/10.1007/s43441-021-00295-8
- Minerva Research Solutions. 2024. SMOs improve clinical trial efficiency. https://minervaresearchsolutions.com/smos-improve-clinical-trial-efficiency/
- Hadzic A, Brathwaite JS, Sheth M, Makutam V, Warne S. 2024. Social media marketing and its vital role in improving clinical trial recruitment. Clinical Researcher 38(6). https://acrpnet.org/2024/12/13/social-media-marketing-and-its-vital-role-in-improving-clinical-trial-recruitment
- Anderson RL, Baber N. 2011. Economic impact of low-enrolling clinical research studies. Journal of Clinical Research. PMC3203249
- Bridging clinical research gaps: How site augmentation enhances trial success. 2025. MedCity News. https://medcitynews.com/2025/01/bridging-clinical-research-gaps-how-site-augmentation-enhances-trial-success/#:~:text=Additionally%2C%20staffing%20turnover%20continues%20to,financial%20system%20to%20benefit%20all

Alen Hadzic, MIE, is CEO of Clinical Trial Scan, a company specializing in patient enrollment.

LaShoun Sanders, BS, is CEO of iHealthClinical, a site management organization.

Justin Scott Brathwaite, MBA, is a Site Readiness and Regulatory Senior Startup Specialist with Fortrea, a contract research organization.



