Clinical Researcher—June 2025 (Volume 39, Issue 3)
PEER REVIEWED
Holly Bookless, BSN, RN, NE-BC; Emily Brown;
Lisa Hafer, MPH, CCRP; Deanna Golden-Kreutz, PhD;
Paula Smailes, DNP, RN, CCRP, ACRP-CP
Now one of the oldest units of its type in the United States, the Clinical Research Center (CRC) at The Ohio State University Wexner Medical Center (OSUWMC) was first established in 1960.{1} As part of an academic medical center (AMC), the unique ability of the CRC to support both inpatient and outpatient protocols of varying complexity contributes to the success of the university’s research mission. Housed within the Center for Clinical Research Management (CCRM), the CRC supports clinical research sponsored by a variety of agencies, including industry and federally funded studies.
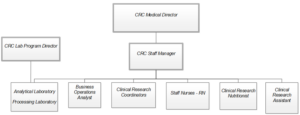
The CRC is a hospital-based unit that averages 230 visits per month. Investigators seek out the nursing, lab, and nutrition core services from the CRC for a variety of reasons. Research procedures including DEXA scanning, bronchoscopies, pharmacokinetic sampling, and administration of investigational medications can be easily executed with the assistance of CRC staff. To implement these protocols, the CRC is composed of a staff of six registered nurses, two study coordinators, two research assistants, four laboratory technicians, a clinical research nutritionist, and a business operations analyst, all of whom report to the CRC staff manager (see Figure 1). This team manages approximately 115 clinical research studies from 51 departments housed within 10 different colleges across the university.
Figure 1: Center for Clinical Research Management’s Clinical Research Center Organizational Chart
During staff meetings, ideas are encouraged to propose what could help run a project or process more smoothly and efficiently. Given the obvious disparity between the number of staff and workload, it is imperative that efficiency in operations be of utmost importance without sacrificing quality with data collection. A customer service approach is used to ensure that the unit meets the needs of investigators and staff to ensure study timelines are met and that their recruited participants are satisfied with the research experience. The CRC had already been using Research Electronic Data Capture (REDCap) for data capture, but thanks to staff innovation, it was determined that many of their antiquated workflows could be efficiently improved by using this tool.
Transitioning CRC Workflows to REDCap
The Ohio State University Clinical and Translational Science Institute (CTSI) houses REDCap as a central location for data processing and management. REDCap provides a secure, web-based application that is flexible enough to be used for a variety of types of research, provides an intuitive interface for users to enter data and has real-time validation rules (with automated data type and range checks) at the time of entry. It offers easy data tracking with audit trails and reporting functionality for monitoring and querying patient records. It also includes an automated export mechanism to common statistical packages.
The literature has shown that REDCap can facilitate research operations at investigational sites beyond its use as a data collection tool for studies. REDCap has been linked to reducing the costs of conducting research, providing efficient data storage, and facilitating research development.{2} It has also been shown that REDCap can be a time-saving tool to track and report ongoing stakeholder engagement.{3} Carvajal et al.{4} created a data management system based on REDCap software as an alternative to a paper-based system that is notoriously a time-consuming practice. Another benefit of REDCap is its sophisticated tracking system, which captures each user’s activity in a complete audit trail{5}; and Dunn et al.{6} discovered the real-time data collection capabilities of REDCap, which, along with its efficient organization and storage of research data, have been critical to advancing research.
With the literature in mind, an evidenced-based project was initiated to capitalize on REDCap to make labor intensive workflows in the CRC more efficient. The utilization of REDCap beyond its electronic data capture feature has been instrumental for the CRC. Innovative workflows have been developed over time to capitalize on many features of the software. As the functions of REDCap became better understood, additional unit inefficiencies were identified and improved upon thanks to REDCap functionality. These automated processes have led to an increase in satisfaction among the CRC team and the investigators and patients using its services.
Improving Satisfaction Using REDCap
Participant Satisfaction
There was a realization that the CRC was not part of the academic medical center’s Press Ganey patient satisfaction surveys; however, there was a desire to better understand satisfaction for those research participants using CRC services. At the time, the CRC received funding from the National Institutes of Health (NIH), which was important because NIH site visits occurred whereby research participant satisfaction data could show quality with operations. A research participant satisfaction survey was initially developed to be disseminated to participants in a paper format using Teleform.
CRC leadership were participating in a Research Subject Satisfaction Taskforce that included other sites around the clinical research enterprise. With this collaboration, a research participant satisfaction survey was developed, placed in REDCap, and housed within what was then known as the Center for Clinical and Translational Science (now the CTSI). A pilot study was developed and approved by the institutional review board, then the Research Participant Satisfaction Survey was tested across participating sites, including the CRC.{7}
A survey link was created specific to the study investigator or research site. For survey completion with REDCap, patient participants would be given an anonymous link with no means for the survey’s creators to determine who completed it.
Consideration was made for the length of time the subject was expected to participate in the study. For example, if the study was five years long, a survey may be given halfway through study participation. Within the CRC, if only one study visit was needed, the survey would be given at the conclusion of CRC study activity. To increase compliance with survey completion, CRC staff would access the survey by anonymous link on a Workspace on Wheels (WOW) and the participant could complete the survey before leaving the facility. To date, analysis of this survey is completed quarterly and consistently shows that the CRC is in the 90th percentile of participant satisfaction across all measurements.
Investigator Satisfaction
While the process of acquiring participant satisfaction data was ongoing, there was an increased focus on understanding the needs of investigators and their study teams, who are also consumers of CRC services. This became important as the CRC expanded its scope across a wide array of therapeutic areas.
The Investigator Satisfaction Survey was placed in REDCap and a link to the survey was e-mailed to both the investigator and study coordinator from the CRC medical director. A focus was made on surveying teams with active studies or those studies closed within the past year. Survey findings showed that the CRC did well in terms of the implementation of protocol activities, responsiveness of staff, and environment for participants. Areas for improvement included study start-up speed, cost of services, and participant scheduling.
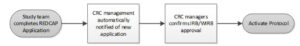
Start-up speed was initially slowed due to the Microsoft Word–based application and the inclusion of a Scientific Advisory Committee, which was previously required when the CRC operated under NIH funding. Adding to this was an inefficient workflow of calls and e-mails that included up to 27 steps from the time of initial investigator outreach to protocol activation. Using REDCap, the process was reduced to four steps (see Figure 2).
Figure 2: Improving Speed to Start with REDCap
When the CRC was supported by the NIH under the General Clinical Research Center (GCRC) grant funding mechanism, the cost of services was provided to investigators free of charge. Once the NIH closed GCRC funding, the CRC was required to adopt a chargeback model to recover the cost of services and provide support for the unit. Studies could no longer use CRC services for free and CRC costs needed to be added to study budgets. This was an adjustment for investigators who, as a result, became more focused on their research questions and what data collection was important.
When the investigator survey showed that customers were not satisfied with the participant scheduling process, a Google calendar was created. This allows study teams to view the CRC’s available hours, showing investigator teams the center’s availability in the moment when participants were being recruited. This saved coordinators several steps in their process, ultimately increasing both study coordinator and participant satisfaction.
Automating Processes Using REDCap
Using REDCap to gather stakeholder satisfaction metrics helped to improve the delivery of CRC services. With further consideration for how REDCap functionality could be capitalized upon for other operational workflows, additional automated workflows were developed (see Table 1). The ability to capitalize on features that assist with the elimination of paper workflows, electronic storage of documents, and automatic notifications proved that it could be used as a tool well beyond research data capture.
Table 1: REDCap Automation Initiatives in the CRC
| Research Initiative | Prior Workflow | Inefficiency | Improved Workflow in REDCap |
| Requests for CRC Services | Paper | Volume burden, paper storage | One-stop shop for requesting study services from start to finish. Contains document repository for important documents. Creates metrics to share with leaders about the utilization of CRC services. |
| Protocol Development | Paper | Volume burden, paper storage | CRC staff use REDCap as checklist for development task, manager notified when development is complete. |
| Rate Quote Requests | Lost to follow-up, hard to organize/report metrics | Provides the investigator a quote during grant writing, then the system is set to automatically ask every six months if CRC services are still needed. | |
| Analytical and Development Lab Request/ Implementation Forms | E-mail to manage requests and intake | Delays waiting for e-mail replies, hard to organize | Automation using a link to REDCap that makes it easier to track the request. Reminders are sent automatically if information is missing. Rate quotes can be approved within the system. Request is tracked through fulfillment. |
| Randomization Notification | CRC staff not informed of randomization outcome in a timely manner | CRC promoted the use of REDCap for randomization. | |
| Invoicing | Excel | Manual process for invoicing. | Central repository for payments with automatic notifications. |
Request for Services
Investigator survey feedback contributed to the transition of CRC request intake process to move from a 10- to 15-page paper template to one page intake form in REDCap. The revised process decreased the time required by investigators to request CRC services as well as the time for staff to review the request. This streamlined the intake process, making it easier and faster for investigators to begin their research using CRC services.
Protocol Development and Management
Prior to using REDCap, there was no central method for ensuring that all phases of the protocol set up process were completed, nor was there a means to assure protocol readiness for each CRC group (nursing/lab/nutrition). Study information and metrics were tracked via Microsoft Word. REDCap solved these problems by providing transparency of study progression and gives the CRC manager a means to track the status of each core in one place.
Once an investigator determines that the CRC will be an important part of study implementation, a request for CRC services must be completed in REDCap. From an administrative perspective, the utilization of REDCap is integral to CRC operations as the first step and continues throughout the protocol life cycle (see Figure 3).
Figure 3: Protocol Life Cycle in the CRC

Quote Requests
Quote requests were previously managed via e-mail and became hard to track. REDCap quote requests include a notification system, informing the appropriate staff. It provides a place for the investigator to upload documents and respond to the template language needed by the CRC.
Only one person could work the request previously, but now in REDCap, the transparency allows the CRC team to follow the progress of the request and to contribute through study execution. REDCap is now the central repository for rate quotes.
Lab Requests
REDCap serves as a central repository for lab services from the initial request for services through study completion. A REDCap survey link allows a requester to provide more detail than can be communicated via e-mail. Lab staff are able to receive quote approval notifications and follow up with investigators via REDCap, including reminder messages if investigators become unresponsive. Multiple staff members can work on the same request simultaneously to ensure continuity of services.
After the quote is approved, the CRC staff can use REDCap to track the next phase of the process, such as implementation, doing the work, ordering kits, or preparing for study start up. Leadership can easily pull reports to ensure study timelines are being met.
Randomization
One protocol was able to capitalize on the randomization feature of REDCap. Doing so provided more transparency and timely notification of the randomization, which promoted timely delivery of research procedures.
Invoicing
Payment tracking is now done in REDCap as a replacement for using Excel spreadsheets. This created a way to organize the finances of core services while tracking net revenue vs. gross, and can be organized by fiscal year. REDCap can easily provide metrics via the reporting feature. Once payment has been received, the historic information can be tracked because it operates as a central repository of payment and funding source.
Conclusion
The improved CRC efficiencies and quality improvement efforts have shown to significantly impact operations by impacting both investigator and research participant satisfaction in a positive way. The value of stakeholder collaboration and feedback shows them that their voice matters and, at the same time, identifies areas of opportunity and improvement. REDCap has been central to these initiatives, along with empowering staff to identify processes that could be streamlined.
While REDCap benefits those who use CRC services, it has also been impactful to unit efficiency. This directly relates to staff engagement with the unit and speaks to the cohesiveness of the unit. By encouraging staff to develop ways to improve the unit, it in turn creates a more positive working environment. Each year the Staff Engagement Survey is distributed to employees by the AMC to determine staff satisfaction with their work. The most recent survey showed that out of 16 CRC staff, 90% find their work to be favorable.
There are considerations with a conversion to REDCap for operations management. The first is availability. Initially REDCap was limited to AMCs, but the scope has widened for those who may use this tool. Secondly, staff education is pertinent to adoption and success. All CRC staff took an introductory REDCap course, so time is needed to ensure that staff are educated in its function. REDCap also has a tutorial that users can follow to learn how to create forms. Use of REDCap operationally is easier than the case is for study protocols, because there is no need to track or retain versions and any team member can make changes.
Over the last 30 years, technology has been infiltrating clinical research operations. CRC staff at The Ohio State University have adapted to not only keep up with industry trends, but to serve as change agents dedicated to both efficiency and quality improvement. As research and technology have evolved, so do CRC operations. If processes are determined to be outdated, staff are encouraged to communicate the identified problem and develop new strategies for success to share with the team.
REDCap has contributed to automated efficiency for the CRC and, overall, REDCap is directly attributed to a better research experience for stakeholders and improved operations for the CRC. More information on the limitations and availability of REDCap can be found at https://project-redcap.org/.
This publication was supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grant Number UM1TR004548. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- The Ohio State University. 2020. Ohio State’s Clinical Research Center: Enabling innovation throughout the university. https://medicine.osu.edu/news/clinical-research-center
- Garcia K, Abrahao A. 2021. Research Development Using REDCap Software. Healthcare Informatics Research 27(4):341–9. doi:10.4258/hir.2021.27.4.341
- Gesell S, Halladay J, Mettam L, Sissine M, Staplefoote-Boynton B, Duncan P. 2020. Using REDCap to track stakeholder engagment: A time-saving tool for PCORI funded studies. Journal of Clinical and Translational Science 4(2):108–14. doi:10.1017/cts.2019.444
- Carvajal C, Vallejos C, Lemaitre D, Ruiz J, Guzman C, Aguilera V, . . . Calligaris S. 2019. A REDCap application that links researchers, animal facility staff and members of the UACUC in animal health monitoring. Laboratory Animals 53(5):500–7. doi:10.1177/0023677218815723
- Harvey L. 2018. REDCap: web-based software for all types of data storage and collection. Nature 56(625) https://www.nature.com/articles/s41393-018-0169-9
- Dunn W, Cobb J, Levey A, Gutman D. 2016. REDCetr: Workflow and tools to support the migration of legacy clinical data capture systems to REDCap. International Journal of Medical Informatics 93:103–10. doi:10.1016/j.ijmedinf.2016.06.015
- Smailes P, Reider C, Hallarn R, Hafer L, Wallace L, Miser W. 2016. Implementation of a Research Participant Satisfaction Survey at an Academic Medical Center. Clinical Researcher 30(3):42–7.
Holly Bookless, BSN, RN, NE-BC, is the Nurse Manager for the Clinical Research Center housed within the Center for Clinical Research Management at The Ohio State University College of Medicine.
Emily Brown is Business Operations Senior Analyst for the Clinical Research Center in the Center for Clinical Research Management at The Ohio State University College of Medicine.
Lisa Hafer, MPH, CCRP, is an Assistant Director of Operations for the Center for Clinical Research Management at The Ohio State University College of Medicine.
Deanna Golden-Kreutz, PhD, is a Senior Director for the Center for Clinical Research Management and Clinical Research Center at The Ohio State University College of Medicine.
Paula Smailes, DNP, RN, CCRP, ACRP-CP, (paula.smailes@osumc.edu) is the Principal Trainer for Clinical Research as a Health Systems Informatics Analyst 4 at The Ohio State University Medical Center and Visiting Professor with Chamberlain College of Nursing.



