Clinical Researcher—August 2022 (Volume 36, Issue 4)
POLICY & PROCEDURE
Daniel Kavanagh, PhD, RAC
Institutional biosafety committees (IBCs) play a critical role in ensuring the safe and responsible conduct of research involving genetically modified products, infectious agents, and biological toxins. For clinical research involving genetically engineered products, IBCs review protocols and procedures to minimize risks to clinical staff, visitors, the general public, and the environment. In doing so, IBCs enhance and promote clinical research and scientific discovery.
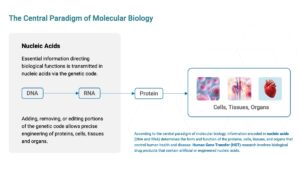
Many aspects of human health and disease are controlled by information encoded in our DNA. To make proteins—the building blocks of cells, tissues, and organs—information from the genetic code is transcribed from DNA into mRNA and translated from mRNA in proteins. Chemically, DNA and RNA are classified as nucleic acids. With modern synthetic technology it is relatively straightforward to synthesize nucleic acids encoding any desired sequence and programmed to alter biological functions. Genetically engineered nucleic acids are being incorporated into an increasing array of medical products designed to treat or prevent disease.
The promise of genetic engineering and synthetic biology has already been demonstrated by more than a dozen gene transfer products—including biologic drugs and vaccines—that have U.S. Food and Drug Administration (FDA) marketing approval. These include cancer-curing chimeric antigen receptor T cell (CAR-T) therapies and mRNA vaccines to prevent infectious disease, among others.
For many of these products, the genetically modified components only persist for a short time in the human body. However, some gene transfer products have a built-in capacity to replicate themselves in a human research participant, and some gene transfer products have the capacity to integrate into chromosomes and make permanent changes in the DNA of affected cells. In some cases, the gene transfer products are living microbes derived from naturally occurring infectious agents.
Assessing potential risks associated with various genetically modified products requires expert knowledge of microbiology, molecular biology, environmental health and safety, and related disciplines. Biosafety is the field of practice dedicated to managing the risks of accidental exposure to genetically modified products and infectious agents in clinical and nonclinical research.
To promote proper oversight of research with genetically modified products, the National Institutes of Health (NIH) published the first version of the current NIH Guidelines for Research Involving Recombinant and Synthetic Nucleic Acids (NIH Guidelines){1} in 1976, which include instructions defining the purpose, responsibilities, and composition of IBCs. Clinical research subject to the NIH Guidelines must be approved by an IBC prior to initiation.
For clinical research, IBC membership should include experts in domains such as microbiology, biosafety, and genetic engineering. In addition, each IBC must include two community members who are unaffiliated with the research site, who live or work nearby, and who can represent community interests and values on the IBC. Each IBC must be registered with the NIH, and each registration applies to one specific institution. There are currently more than 2,700 IBCs registered with the NIH.
IBCs in Clinical Trials
Under the NIH Guidelines, IBC review is required for many kinds of basic science and translational research, as well as clinical trials; this column focuses on oversight of clinical research. Clinical trials requiring IBC review fall into the category of human gene transfer (HGT) research. A technical definition of HGT research is provided in the NIH Guidelines Section III-C, but essentially HGT research is the introduction into a human research participant of a product containing genetically engineered or artificially modified DNA or RNA (with certain exceptions for molecules that are very small and incapable of inducing lasting molecular changes in the cell).
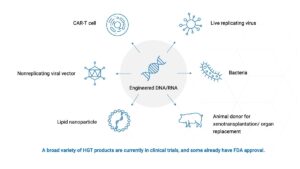
Examples of HGT products include: an mRNA or DNA vaccine; a gene therapy delivered by an engineered “viral vector”; a lymphocyte engineered to kill cancer (e.g., CAR-T or CAR-NK therapies); and a bacterial strain genetically modified to express a therapeutic protein in the human gut. Under the NIH Guidelines, IBC approval of HGT research is required when certain types of NIH funding apply to the study. This includes funding for product development, funding to the research sponsor, and/or funding to the research site (including for unrelated research). In addition, the NIH Guidelines recommend IBC oversight of HGT research even when not required due to funding (“voluntary compliance”).
Interventional clinical trials in the United States require approval by an institutional review board (IRB). Clinical trials subject to the NIH Guidelines require review by an IBC in addition to IRB review. IRB review is focused on protection of human research participants, while IBC review is focused on protection of staff, visitors, the general public, and the environment at a clinical trial site.
In the past, there was significant overlap in what was reviewed by IRBs and IBCs with respect to human research participant protection, but under the most recent amendment to the NIH Guidelines, the separation of IRB and IBC responsibilities is much more clearly delineated. Notably, IBCs are no longer required to review informed consent or other participant-facing documents. Nevertheless, IRBs and human research participant protection departments often rely on the expertise of IBC members to address complex risks in gene transfer trials.
Approval by both the IRB and IBC is required prior to initiation of clinical research subject to the NIH Guidelines. IRB and IBC review may be conducted sequentially or simultaneously according to the policy of the institution or committee administrator.
As mentioned above, each IBC must be registered with the NIH. In the past, each IBC was usually registered and administered locally by the respective research institution. Today, many institutions have IBCs that are registered and administered centrally by a commercial service provider. Centrally administered IBC services are especially critical for research sites that lack the scientific and administrative expertise and personnel to manage their own IBC. In addition, many universities and academic research centers find it useful to have an auxiliary IBC administered by a central service provider for clinical trials, alongside a locally administered IBC for basic science and other research.
When reviewing an HGT trial, an IBC will focus on specific questions mandated by NIH or recommended by the Centers for Disease Control (CDC).{2} These include, for example:
- What is the appropriate biological safety level (BSL) for this research? IBCs must approve research at BSL-1, -2, -3, or -4; clinical trials are generally approved at BSL-1 or BSL-2. The BSL designation helps inform the selection of equipment and procedures suitable for safe handling of investigational products.
- What infectious agents or biological toxins may be contained in or produced by the test article/drug product? What measures are in place at the research site to contain these agents and factors?
- Does the site propose to use appropriate equipment? For some trials, a biological safety cabinet is recommended and the site must show that the cabinet is inspected and certified for proper function.
- How are hazardous spills deactivated/disinfected? IBCs ensure that appropriate disinfectants for specific categories of agents are selected from approved lists published by the Environmental Protection Agency.
- How is biohazardous waste disposed of? Biohazardous waste must be appropriately segregated from nonhazardous waste and deactivated or transferred to a qualified waste hauler.
- What measures are in place to minimize the risk of needlestick exposures to experimental drug products? Needlestick injuries are a frequent cause of accidental exposures—the risk of such exposures can be mitigated with appropriate equipment and training.
- How are staff trained and informed on standard operating procedures and emergency response?
Importantly, new developments in fields such as genetic engineering, synthetic biology, and xenotransplantation are constantly raising challenging new questions, so it’s important that IBC members and advisors stay abreast of rapidly progressing discoveries and techniques.
Getting Started with Gene Transfer Research
Gene transfer research represents a rapidly growing sector of clinical drug development in a diverse array of therapeutic areas. Research sites interested in getting involved may download and study the relevant NIH and CDC guidances required to staff, register, and operate a locally administered IBC. On the other hand, commercial services staffed by dedicated biosafety professionals and compliance experts are also readily available to provide IBC oversight services to research sites. In many cases, all costs associated with these services are borne by the commercial clinical trial sponsors. Clinical research sites planning new construction or opening new clinics or pharmacies should engage with their IBC or with a professional biosafety consultant in advance to assess what type of facilities and equipment will best enable engagement with cutting-edge clinical trials.
Conclusion
Many of the most exciting new developments in clinical drug development in the coming years will involve gene transfer research. Contract research organizations, clinical research sites, and investigators can enhance their capabilities and help ensure the safe conduct of research by engaging with a registered IBC and partnering with biosafety experts before, during, and after each new HGT trial.
References
- NIH Guidelines for Research Involving Recombinant and Synthetic Nucleic Acids. https://osp.od.nih.gov/biotechnology/nih-guidelines/
- Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition. cdc.gov/labs/BMBL.html

Daniel Kavanagh, PhD, RAC, (dkavanagh@wcgclinical.com) is Senior Scientific Advisor, Gene Therapy, at WCG and serves as scientific lead in WCG’s IBC Services division.



