Clinical Researcher—December 2024 (Volume 38, Issue 6)
SPECIAL FEATURE
Alen Hadzic, MIE; Justin Scott Brathwaite, MBA; Milan Sheth, MS; Viswakanth Makutam, PharmD, MS; Sandra Warne, BS
Why is marketing and advertising often overlooked in clinical research? Patient recruitment and retention in clinical research frequently encounter obstacles, resulting in delays and increased expenses. While marketing and advertising have the potential to address these issues, they are frequently underutilized in clinical trials due to ethical concerns, regulatory constraints, and misconceptions about their role. Researchers often express concern that incorporating marketing strategies might compromise the trial’s scientific integrity or conflict with regulatory standards that emphasize patient protection over promotional activities.{1} Additionally, a common misconception is that marketing pertains only to commercial products, not medical research.{2} As a result, these tools are often not seen as integral to the clinical trial process.
However, the absence of effective marketing can have real consequences. Lack of targeted outreach can hinder studies from recruiting and retaining diverse patient populations, potentially resulting in biased outcomes and limited generalizability.{3} Incorporating marketing techniques—such as digital recruitment strategies, predictive enrollment timelines, and community engagement—can improve patient diversity, streamline recruitment processes, and ultimately help researchers meet their enrollment goals.{4} Thus, to enhance the effectiveness of clinical trials, it is crucial to draw upon expertise from business strategy, management, marketing, and sales, rather than relying solely on traditional clinical practices.{5}
This article explores how marketing, when applied strategically, can bridge business strategies with clinical objectives to improve patient engagement, enhance recruitment, and ensure more timely clinical trial outcomes.
The Power of Predictive Timelines in Digital Recruitment
Clinical trial sponsors often overlook the value of digital marketing, social media, and other outreach methods in connecting with potential participants. Conventional recruitment techniques like flyers, physician referrals, and cold calls often prove to be slow, inefficient, and unreliable–which contribute to delays that adversely impact timelines and study budgets.{6} However, digital platforms like Facebook and Instagram offer social media targeting that presents a solution for any recruitment campaign.{6} Such platforms also provide a data-driven approach to calculate enrollment fulfillment timelines with quantifiable and actualized precision, thus allowing for real-time adjustments in targeting and strategy.
Social media targeting can streamline recruitment and allow for precise estimation of timelines for enrollment. Facebook and Instagram offer unparalleled targeting options that make traditional methods obsolete. Utilizing these platforms enables clinical trial recruitment teams to focus on potential participants based on demographics such as age, sex, and proximity to research site through radial targeting.
There are numerous ways to engage potential participants, allowing for multiple targeting strategies that can be tested simultaneously. Real-time metrics provide essential information on the effectiveness of various strategies in engaging the intended audience and optimizing cost-per-interest. This flexibility enables adjustments to be made in real time, allowing for budget optimization and strategic reallocation based on the relevance, cost, and overall performance of each targeted interest.
A lead-generation strategy is one of the simplest and most cost-effective targeting methods. By creating an advertisement that directs potential participants to a questionnaire tailored to the study protocol, this approach ensures only qualified individuals proceed. The questionnaire uses conditional formatting to filter and register only those who meet the criteria as potential leads. This strategy effectively identifies interested and qualified patients residing within a defined radius of the clinical site, enhancing the recruitment process.{7}
Once a targeted digital campaign is live, it becomes possible to estimate the timeline for enrollment fulfillment by leveraging data from ad performance and pre-screening processes. Social media platforms provide real-time metrics that reveal how many individuals are clicking on ads, completing pre-screening questionnaires, registering as leads, and expressing interest in the study. As more leads are registered within a campaign, the cost per lead conversion generally decreases, and the overall performance becomes more efficient. This unique aspect of online advertising ensures that, for successful campaigns, costs typically decrease the longer the campaign runs.
When leads begin registering, native advertising algorithms optimize by targeting similar profiles to those already qualified, resulting in an increasingly refined “cost per lead.” This cost often decreases over time as the algorithm gains insights into the ideal patient profile. Evaluating these data points allows for the prediction of potential participants entering the recruitment funnel and estimation of both the timeline and necessary costs to generate a specified number of prescreened leads. Once there are data available on how leads transition from the digital ad stage to pre-screening calls, it becomes possible to develop an enrollment conversion model for more precise projections.
For instance, if it is known that 80% of the pre-screened leads generated through social media result in successful pre-screening calls, 60% of these leads book appointments to visit the research site, 70% of those actually attend the appointment, and 60% of attendees ultimately enroll in the study, you can accurately determine how many pre-screened leads are needed to enroll 50 patients. Based on these conversion rates, to achieve 50 enrolled patients, you would need approximately 248 prescreened leads.
Target Number of Enrolled Patients: The number of patients you want to enroll.
Conversion Rate 1: The percentage of pre-screened leads resulting in successful pre-screening calls.
Conversion Rate 2: The percentage of leads who book appointments.
Conversion Rate 3: The percentage of booked appointments that actually attend.
Conversion Rate 4: The percentage of attendees who ultimately enroll.
Additionally, if the cost per lead is $50, the total cost to generate these 248 leads would be $12,400. With this investment, the 50 required patients can be enrolled, resulting in a cost per enrolled patient of $248. By knowing these key conversion metrics and costs, one can precisely predict how many leads are necessary, the total budget required, and the cost per patient for enrollment, allowing for better planning and allocation of resources in clinical trial recruitment.
Digital advertising also allows for continuous optimization throughout the recruitment process, which is critical for staying on track with enrollment goals. Two key re-optimization points can help refine the process.
Re-Optimization Point #1: Prescreening Adjustments
After initial calls, if it is found that many participants are disqualified due to missing medical history or incorrect criteria, you can adjust your questionnaires or messaging to attract more suitable candidates. This prevents wasted time and money on advertising for unqualified leads and helps keep the recruitment timeline on track. You simply need to turn the advertisement off, edit it, and turn on the new one. This process is live and immediate, with no need to wait.
Re-Optimization Point #2: Onsite Screening Adjustments
If participants are frequently disqualified during onsite screenings, feedback from the clinical team can help refine your targeting. For instance, adjusting geographic targeting or including additional medical history questions in the digital ads can help reduce disqualifications, ensuring that only qualified candidates move forward.
One of the most powerful aspects of social media targeting in clinical trial recruitment is the ability to predict the diversity profile of your participant population with remarkable accuracy. This is relevant because diversifying clinical trial participants is one of the industry’s biggest challenges that requires a multifaceted approach, including marketing and advertising.{8}
By understanding the demographics of the disease, user base of platforms like Facebook and Instagram, and those of the targeted geographic, you can forecast the diversity profile of your enrolled participants. No other method offers this level of precision.
For example, if you’re recruiting for a diabetes trial, you already know that certain populations are disproportionately affected by the disease. In the U.S., studies show higher prevalence rates among Hispanic, African American, and Native American communities.{9} Urban areas with diverse populations, for instance, allow you to adjust your ad radius and messaging to ensure that clinical teams not only reach a broad spectrum of participants, but also recruit from a demographic pool that reflects the diversity necessary for real-world trial outcomes. The interaction between the advertisement and the diversity of the population within the set radial target cannot be replicated by any other method to the authors’ knowledge.
In addition, geographic limitations present significant barriers to the enrollment of diverse and underrepresented populations in clinical trials, as these groups may not have easy access to trial sites.{10} However, targeted marketing and advertising strategies offer a possible solution by reaching and engaging individuals from specific backgrounds who live near the trial locations.
Thus, by combining disease state demographics, social media platform user data, and regional demographic information, you can predict and achieve a representative participant pool. This level of control over your diversity profile is unique to digital recruitment methods and cannot be replicated with traditional approaches.
Crafting Predictive Enrollment in Four Steps
Once you’ve collected data on your conversion rates, lead generation rates, and disqualification rates, you can build a predictive timeline for enrollment fulfillment in four steps.
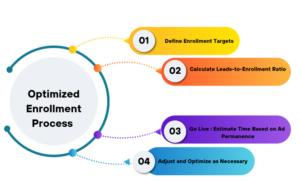
Step 1: Define Enrollment Targets
Start by determining how many enrolled participants are needed to complete the study. Let’s take the previous case of 50 enrolled patients.
Step 2: Calculate Lead-to-Enrollment Ratio
Next, use historical data to calculate how many leads are required for each enrollment. Based on the previous example, 80% of pre-screened leads result in successful pre-screening calls, 60% of those book appointments, 70% of those attend the appointment, and 60% of attendees enroll. With these conversion rates, you would need approximately 248 pre-screened leads to enroll 50 patients.
Step 3: Go Live
Here, you estimate time based on ad performance. Review the ad campaign’s daily performance to determine how quickly these 248 leads can be generated. If the ad campaign generates 10 leads per day, it would take about 25 days to gather the 248 required leads to meet the enrollment goal.
Step 4: Adjust and Optimize as Necessary
Finally, account for potential delays in the process, such as the need to re-optimize the campaign or adjust prescreening questions based on real-time feedback. This could add a few extra days to the initial timeline, but these adjustments will improve participant quality and ensure higher enrollment rates. Conversely, by optimizing the ads, a few days may even be removed from your timeline. Factoring in these delays and incorporating important feedback will give a more accurate and optimized timeline for achieving recruitment goals.
By following these steps, one can more effectively plan and execute clinical trial recruitment, ensuring that enrollment targets are met within a realistic timeframe and budget.
Concluding Remarks
In summary, social media targeting and data-driven recruitment have revolutionized the way clinical trials are conducted, offering precision, inclusivity, and efficiency that traditional methods simply cannot provide. Leveraging real-time feedback loops and continuously refining the recruitment process can allow for greater accuracy when predicting enrollment fulfillment timelines, helping to ensure that a study stays on track.
Credits
The authors would like to thank the following organizations: VAST Clinical Research, LLC; Optimal Research Sites LLC; ClinMastery, LLC; and Chemidox Clinical Trials, Inc.
References
- Muench F, et al. 2018. Barriers to effective recruitment in clinical trials: The role of marketing. Journal of Clinical Research 56(2):190–8.
- National Institutes of Health. 2023. Social Media and Patient Recruitment in Clinical Research. https://www.nih.gov
- Gray R, et al. 2020. Recruitment and retention in clinical trials: The ethical and practical challenges. Journal of Clinical Trials 45(3):215–24.
- Zarin DA, et al. 2017. Digital tools for clinical trial recruitment: A review. Clinical Trials 14(6):530–9.
- Francis D, Roberts I, Elbourne, DR, et al. 2007. Marketing and clinical trials: A case study. Trials 8:37. https://doi.org/10.1186/1745-6215-8-37
- Topolovec-Vranic J, Natarajan, K. 2016. The Use of Social Media in Recruitment for Medical Research Studies: A Scoping Review. Journal of Medical Internet Research 18(11):e286.
- Whitaker C, Stevelink S, Fear N. 2017. The Use of Facebook in Recruiting Participants for Health Research Purposes: A Systematic Review. J Med Internet Res 28;19(8):e290. doi: 10.2196/jmir.7071. PMID: 28851679; PMCID: PMC5594255.
- Brathwaite J, Wolgast M, Bickhart, S. 2023. Viable Strategies to Increase Clinical Trial Patient Diversity. AL-Kindy College Medical Journal 19(2):3–6. https://doi.org/10.47723/kcmj.v19i2.1106
- Chow EA, Foster H, Gonzalez V, McIver L. 2012. The Disparate Impact of Diabetes on Racial/Ethnic Minority Populations. Clin Diabetes 30(3):130–3. https://doi.org/10.2337/diaclin.30.3.130
- Brathwaite J, Goldstein D, Dowgiallo E, Haroun L, Hogue K, Arakaki T. 2024. Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations. Clinical Researcher 38(2). https://acrpnet.org/2024/04/12/barriers-to-clinical-trial-enrollment-focus-on-underrepresented-populations
Alen Hadzic, MIE, is CEO of Clinical Trial Scan.
Justin Scott Brathwaite, MBA, is a Site Readiness and Regulatory Senior Specialist.
Milan Sheth, MS, is a Clinical Research Coordinator.
Viswakanth Makutam, PharmD, MS, is a Clinical Research Quality Associate.
Sandra Warne, BS, is a Clinical Project Manager.



